Cellular Communication in Cancer
 The Cellular Communication in Cancer Program explores the molecular signals within and between cells that drive cancer. Researchers in this Program are developing innovative new models for human tumors and advanced imaging technology with a goal of identifying potential “druggable” targets and mechanisms of drug resistance in cancer. Current research is focused on identifying and targeting the intracellular signal transduction mechanisms in cell communication; cell-cell and cell-extracellular matrix (ECM) communication in the microenvironment; and communication at the whole-body level between tumor and host.
The Cellular Communication in Cancer Program explores the molecular signals within and between cells that drive cancer. Researchers in this Program are developing innovative new models for human tumors and advanced imaging technology with a goal of identifying potential “druggable” targets and mechanisms of drug resistance in cancer. Current research is focused on identifying and targeting the intracellular signal transduction mechanisms in cell communication; cell-cell and cell-extracellular matrix (ECM) communication in the microenvironment; and communication at the whole-body level between tumor and host.
Program Co-leaders
Linda Van Aelst, Ph.D.
Corina Amor Vegas, M.D., Ph.D.
![]() The Cellular Communication in Cancer Program has three overarching themes: (I) identifying and targeting signaling in cancer using advanced model systems; (II) elucidating and targeting tumor-host interactions, and (III) understanding cancer in the context of the whole-body. Members of the Program include experts with an in-depth understanding of different families of signaling proteins, integrated with investigators who are leaders in the fields of cancer biology and metastasis, and complemented by investigators who innovate technologies for studying molecular and cellular functions. As such, the Program generates basic discoveries that can drive new areas for cancer therapy. Work of the Program members is heavily dependent on the support from the CSHL Cancer Center Shared Resources, in particular the Animal, Animal & Tissue Imaging, Antibody & Phage Display, Mass Spectrometry, Flow Cytometry, and Sequencing Technologies & Analysis Shared Resources.
The Cellular Communication in Cancer Program has three overarching themes: (I) identifying and targeting signaling in cancer using advanced model systems; (II) elucidating and targeting tumor-host interactions, and (III) understanding cancer in the context of the whole-body. Members of the Program include experts with an in-depth understanding of different families of signaling proteins, integrated with investigators who are leaders in the fields of cancer biology and metastasis, and complemented by investigators who innovate technologies for studying molecular and cellular functions. As such, the Program generates basic discoveries that can drive new areas for cancer therapy. Work of the Program members is heavily dependent on the support from the CSHL Cancer Center Shared Resources, in particular the Animal, Animal & Tissue Imaging, Antibody & Phage Display, Mass Spectrometry, Flow Cytometry, and Sequencing Technologies & Analysis Shared Resources.
At the Lab: Catch me if you cancer
June 3, 2025
You’ve seen her on FOX and the cover of Newsday. Now, hear about the knowledge gaps that make her discovery so crucial and the noble goals driving it.
At the Lab: A vitamin for prostate cancer
April 8, 2025
For the Season 2 premiere of our podcast, we sit down with CSHL Professor Lloyd Trotman to discuss what could be a major breakthrough in men’s health.
Are pro-oxidants anti-prostate cancer?
January 7, 2025
New research from CSHL Professor Lloyd Trotman offers men of all ages a look inside the possible future of prostate cancer treatment and prevention.
Vitamin K supplement slows prostate cancer in mice
October 24, 2024
The finding from CSHL Professor Lloyd Trotman and his team sets the stage for pilot studies in human prostate cancer patients.
A century of women’s health breakthroughs
July 22, 2024
Oscar Riddle identified the hormone behind lactation in 1933. The discovery at CSHL continues to inspire research on women’s health and breast cancer.
A link between breast changes and . . . UTIs?
May 2, 2024
CSHL breast cancer researchers, led by Associate Professor Camila dos Santos, made a surprise discovery while investigating urinary tract infections.
New antibody could target breast cancers
October 30, 2023
CSHL Professor Nicholas Tonks’ team has discovered a new way to target PTPRD, an enzyme that may help some breast cancers spread.
Can our own immune system fight off cancer?
November 10, 2021
Researchers have studied the link between cancer and the immune system for centuries. Today, immunology provides promising solutions to combat cancer.
Why pancreatic ductal adenocarcinoma is so lethal
May 19, 2020
CSHL researchers discovered factors that allow a pancreatic cell to lose its identity and turn into an aggressive cancer cell.
Turning off the immune system is hard. Turning it on against cancer is easier.
January 10, 2018
How Professor Doug Fearon’s experiences with autoimmune disease patients contributed to the discovery of a new potential immunotherapy for cancer.
Three Strohm Sisters Family Foundation donates $5,000 for research on lung cancer recurrence
January 8, 2018
A representative of the Three Strohm Sisters Family Foundation gave $5,000 for lung cancer recurrence research in Mikala Egeblad's lab.
One experiment: Bacteria-trapping DNA webs are repurposed by cancer cells
October 19, 2017
Associate Professor Mikala Egeblad discovers that neutrophil extracellular traps (NETs) help cancer by creating holes in the tissue.
Halfway around the world, a reunion of friends opens door to a cancer discovery
August 31, 2017
After interviewing for a position in a pancreatic cancer lab at Cold Spring Harbor Laboratory, 7,000 miles from his hometown in South Korea, Chang-il.
Long-sought mechanism of metastasis is discovered in pancreatic cancer
July 27, 2017
An epigenetic factor reprograms gene enhancers, enabling cancer cells to “remember” an earlier developmental state and causing metastasis.
Newly discovered mutations impair key cell pathways in pancreatic cancer
May 8, 2017
Researchers have found important new clues to the development of pancreatic cancer.
Pershing Square Sohn cancer research prize awarded to CSHL’s Dr. Mikala Egeblad
May 8, 2017
Cold Spring Harbor Laboratory (CSHL) Associate Professor Mikala Egeblad, Ph.D., has been awarded the Pershing Square Sohn Prize.
Alexa DeAngelis didn’t see a place for herself in science, so she’s making one
April 28, 2017
For Alexa DeAngelis, who was recently awarded a Fulbright scholarship, combining a desire to help people with a passion for biochemistry means design.
New research explains why even targeted therapies eventually fail in lung cancer
March 29, 2017
Dr. Sordella and her team propose a novel theory of how some cancers circumvent the killing power of targeted therapies.
Discovery of distinct cell subtypes around tumors helps explain why pancreatic cancer is so hard to treat
February 23, 2017
Researchers finds that stroma,“wound”-like tissue that surrounds the tumors, helps explain why pancreatic cancer is so resistant to treatment.
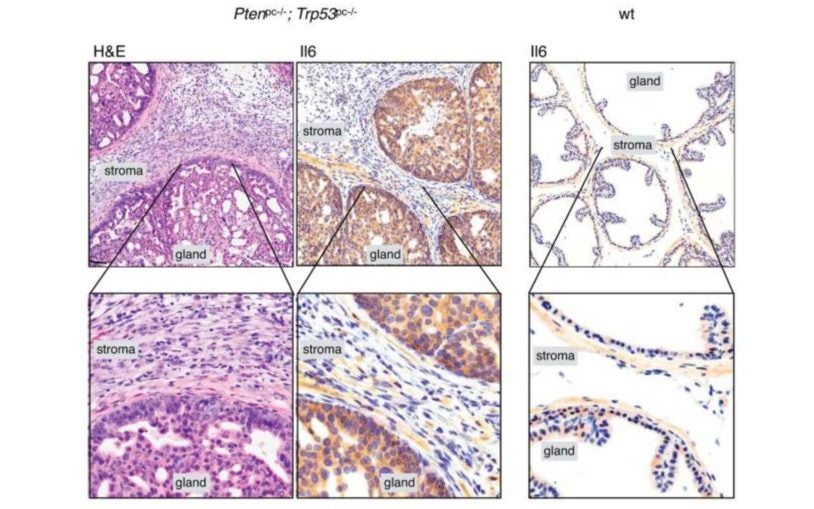
Researchers identify “Achilles’ heel” of PTEN that helps drive prostate cancer progression
February 13, 2017
Researchers at CSHL have discovered that a protein called Importin-11 protects the anti-cancer protein PTEN from destruction by transporting it.
Researchers identify potentially druggable mutant p53 proteins that promote cancer growth
December 9, 2016
Truncated p53 proteins, presumed unimportant, now point to new drug targets for some of "the hardest cancers"
When antioxidants are pro-cancer
November 15, 2016
For Pancreatic Cancer Awareness Month, Base Pairs looks at the science of antioxidants and cancer.
No (real) moustache required to join the “Movember” party
November 15, 2016
Members of Trotman don fake moustaches in order to raise money for prostate cancer research during Movember.
Pancreas and colon tumors reprogram the liver, causing wasting and short-circuiting body’s immune response
November 8, 2016
Research reveals a mechanism that causes wasting in cancer and tests a way to reverse it.
Our most common infection-fighting white blood cells can be hijacked to support cancer spread
October 19, 2016
Neutrophils, the most common type of white blood cell, can be taken over by cancer cells and used as an aid in metastasis.
What’s the connection between antioxidants and cancer?
October 14, 2016
David Tuveson discusses the relationship between antioxidants and cancer, and how the former doesn't necessarily prevent the latter.
Novel drug therapy kills pancreatic cancer cells by reducing levels of antioxidants
July 28, 2016
Researchers demonstrate that in the specific context of pancreatic cells leading to cancer, raisin antioxidant levels should not be raised.
Discovery of new ovarian cancer signaling hub points to target for limiting metastasis
July 10, 2016
A team of researchers at Cold Spring Harbor Laboratory discovers new insights into signaling events that underlie metastasis in ovarian cancer cells.
How healthy cells might help cancer survive
February 4, 2016
A story on Mikala Egeblad's breast cancer research and the importance of the tumor microenvironment.
Masthead Cove Yacht Club raises over $7000 for CSHL cancer research at annual race
November 25, 2015
Members of the Masthead Cove Yacht Club (MCYC) raised $7,124 from their annual Masthead Race.
Joni Gladowsky Breast Cancer Foundation donates $80,000 to Cold Spring Harbor Laboratory
July 24, 2015
These proceeds will support breast cancer research in the laboratory of CSHL Professor Nicholas Tonks.
An immune system marker for therapy-resistant prostate cancer
June 4, 2015
Research shows how signaling by an immune system component appears to play an important role in driving particularly aggressive prostate cancer
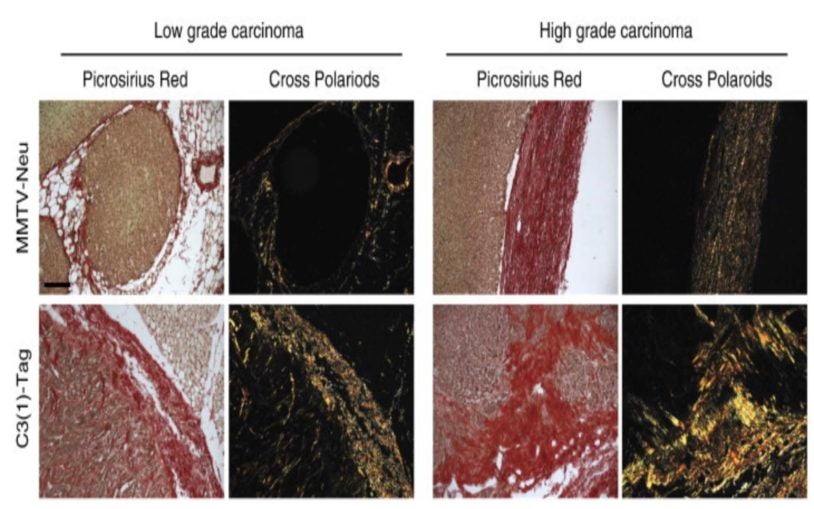
Tumor surroundings are shown to affect progression of different cancer subtypes
May 27, 2015
Researchers discover that cancer progress differently based on characteristics of the tumor microenvironment.
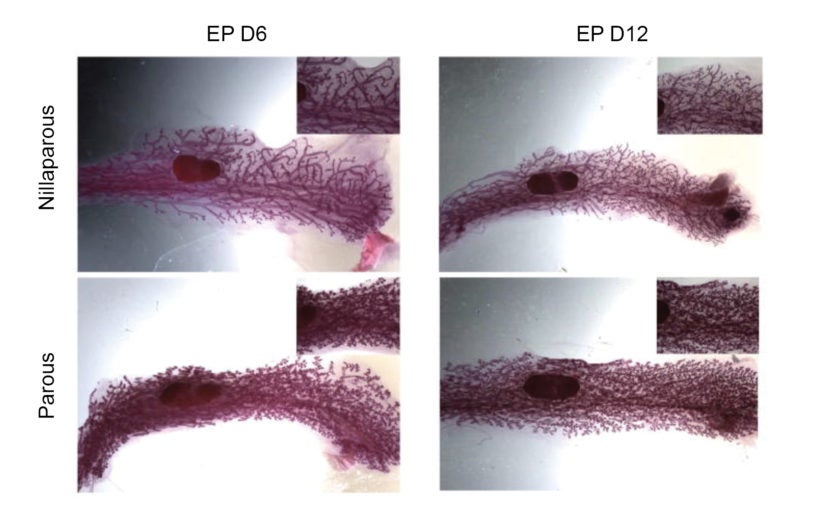
Scientists show the mammary gland ‘remembers’ prior pregnancy, spurring milk production
May 7, 2015
Anecdotal reports of nursing mothers have long suggested that giving milk is a lot easier in second and subsequent pregnancies.
New signaling pathway discovered in HER2-positive breast cancer, and two potentially powerful drug targets
April 20, 2015
Rsearchers discovered a new pathway, which contains at least two potentially powerful drug targets
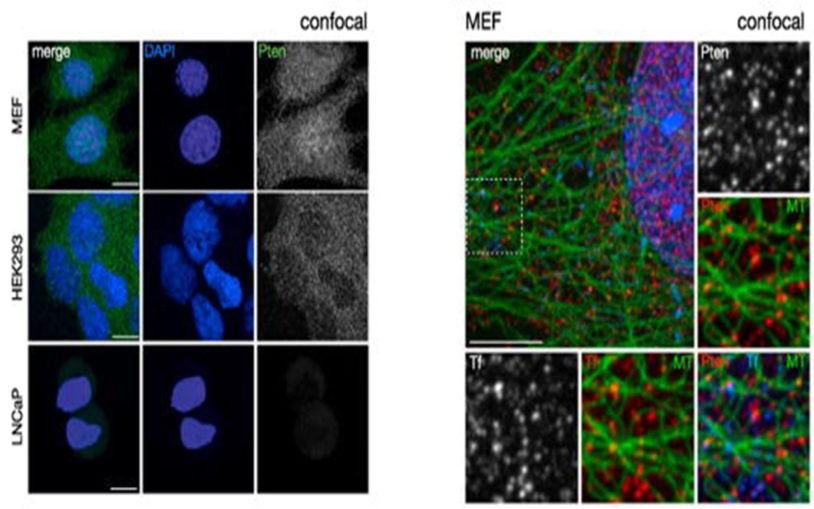
Study revises standard theory of how PTEN, a critical tumor suppressor, shuts off growth signals
April 9, 2015
Scientists discover new evidence explaining precisely how the protein encoded by PTEN (called PTEN) works
One experiment: Cellular highways carry anti-cancer cargoes
April 9, 2015
The gene called PTEN is one of the most important of the body’s natural tumor suppressors.
3D culture system for pancreatic cancer has potential to change therapeutic approaches
January 15, 2015
Organoid technology with human tissue provides a model for full progression of the disease
A novel biomarker for mutant p53 could help pathologists assessing tumors during surgery
January 5, 2015
New discovery may make it easy for cancer surgeons to determine if a patient has a potentially lethal mutation.
 Building publication list.
Building publication list.
Corina Amor Vegas
As we age our body accumulates damaged “senescent” cells that our immune system is no longer able to effectively eliminate. Senescent cells are responsible for the development of aging and age-related diseases like cancer or fibrosis. My group studies how senescent cells evade the immune system thereby identifying new therapeutic approaches.

Jeremy C. Borniger
Patients with cancer frequently experience debilitating symptoms that can impair quality of life and reduce odds of survival. These include drastic changes in appetite, sleep/wake cycles, cognitive function, and pain, among others. Our lab aims to uncover mechanistic interactions between the brain and cancer that drive these phenomena. Reciprocally, we investigate how manipulation of specific brain circuits influences cancer processes in the body.

Lucas Cheadle
The trillions of connections between brain cells enable complex thought and behavior. These connections are wired with great precision through both genetics and in response to an organism’s experiences. Our lab seeks to understand how experiences engage specialized immune cells called microglia to shape the connectivity and function of the brain. We are further interested in how impairments in these processes can contribute to neurodevelopmental disorders such as autism.

Paolo Cifani
We develop innovative mass spectrometry-based approaches to measure how protein activities are regulated under physiologic conditions and in pathological states.

Hiro Furukawa
The nervous system transmits information by passing chemical signals from one nerve cell to the others. This signal transmission relies on a variety of proteins to receive and transmit the chemical signals. My group studies the structure and function of neurotransmitter receptors and ion channels that regulate fundamental neuronal activities.

Michael Lukey
Tumor growth depends upon cancer cells acquiring nutrients from their environment and using these molecules to fuel proliferation. My group studies the nature and regulation of metabolic adaptation during tumorigenesis and metastasis, with the intention of identifying metabolic vulnerabilities that can be targeted for cancer therapy.

Scott Lyons
I provide collaborative research support to CSHL researchers in the area of preclinical in vivo imaging. This includes access to a comprehensive range of imaging modalities, as well as provision of experimental guidance, training and imaging reagents. In addition, my lab develops new and impactful ways to image aspects of in vivo tumor biology that are broadly relevant to the development of new therapeutics and the research interests of the CSHL Cancer Center.

John Moses
My group uses click chemistry to study biological systems at the molecular level. We develop and exploit powerful bond-forming click reactions that enable the rapid synthesis of small functional molecules, including cancer drugs and chemical probes. We apply these novel molecular tools in multidisciplinary discovery projects spanning the fields of biology and chemistry.

Jon Preall
Developing single-cell genomics technologies for applications related to cancer progression, immune surveillance, and discovery of rare novel cell types and transcriptional programs.

Nicholas Tonks
Cells must constantly react to what is happening around them, adapting to changes in neighboring cells or the environment. I study the signals that cells use to exchange information with their surroundings. Our group is finding drugs that target these signals and thus can treat diabetes, obesity, cancer, and autism spectrum disorders.

Kevin Tracey
The major focus of my research is the molecular basis of inflammation and identifying the mechanisms by which neurons control the immune system.

Lloyd Trotman
We pioneered generation of a unique genetic mouse model for therapy and analysis of metastatic prostate cancer. Recently, we developed 3-dimensional whole organ imaging technology that allows us to visualize cancer and metastatic progression in its native environment and at single cell resolution. Now, we use this platform to understand the role of nerves in tumor metastasis, and to develop novel therapeutic interventions against lethal disease.

David Tuveson
Pancreatic cancer is an extremely lethal malignancy. On average, patients who are diagnosed with pancreatic cancer succumb to the disease within 6 months. Research is the only way to defeat pancreatic cancer. My lab is making progress toward finding a cure by detecting the disease earlier and designing novel therapeutic approaches.

Linda Van Aelst
Normal cell function relies on coordinated communication between all the different parts of the cell. These communication signals control what a cell does, what shape it takes, and how it interacts with other cells. I study these signaling networks to understand how they guard against cancer and neurological disorders.

Erika Tse-Luen Wee
Develop and implement state-of-the-art fluorescence imaging and analysis techniques to quantify cell and tissue samples' structure and function.

Johannes Yeh
Cells orchestrate proteins to conduct cell-cell communications and environment sensing in order to execute physiological functions. My lab investigates the mechanisms by which dysregulated signals cause diseases such as cancer, and we are developing therapeutics based on these mechanisms.

Lingbo Zhang
The research in the Zhang laboratory centers on normal and malignant stem and progenitor cells in the hematopoietic system and decodes the role of metabolites in the tumor microenvironment, including nutrients and neurotransmitters, and their genetic effectors in regulating hematologic malignancies. The ultimate goal is to understand how environmental signals such as dietary and neuronal activities regulate stem and progenitor cell development and cancers.