Base Pairs podcast
Fighting cancer is so difficult in part because the healthy cells we want to support often end up casualties in the crossfire of toxic treatments. This episode of Base Pairs is about how we might overcome this obstacle even in some of the most difficult cases: patients with pancreatic cancer. Of all major cancers, pancreatic has the lowest survival rate, because patients are usually too sick to be helped by conventional therapies by the time they’re diagnosed. But recent research on antioxidant levels in the cells of pancreatic cancer patients is homing in on a new, safer avenue for treatment—and it’s not what you’d think based on the reputation antioxidants have gained in popular culture.
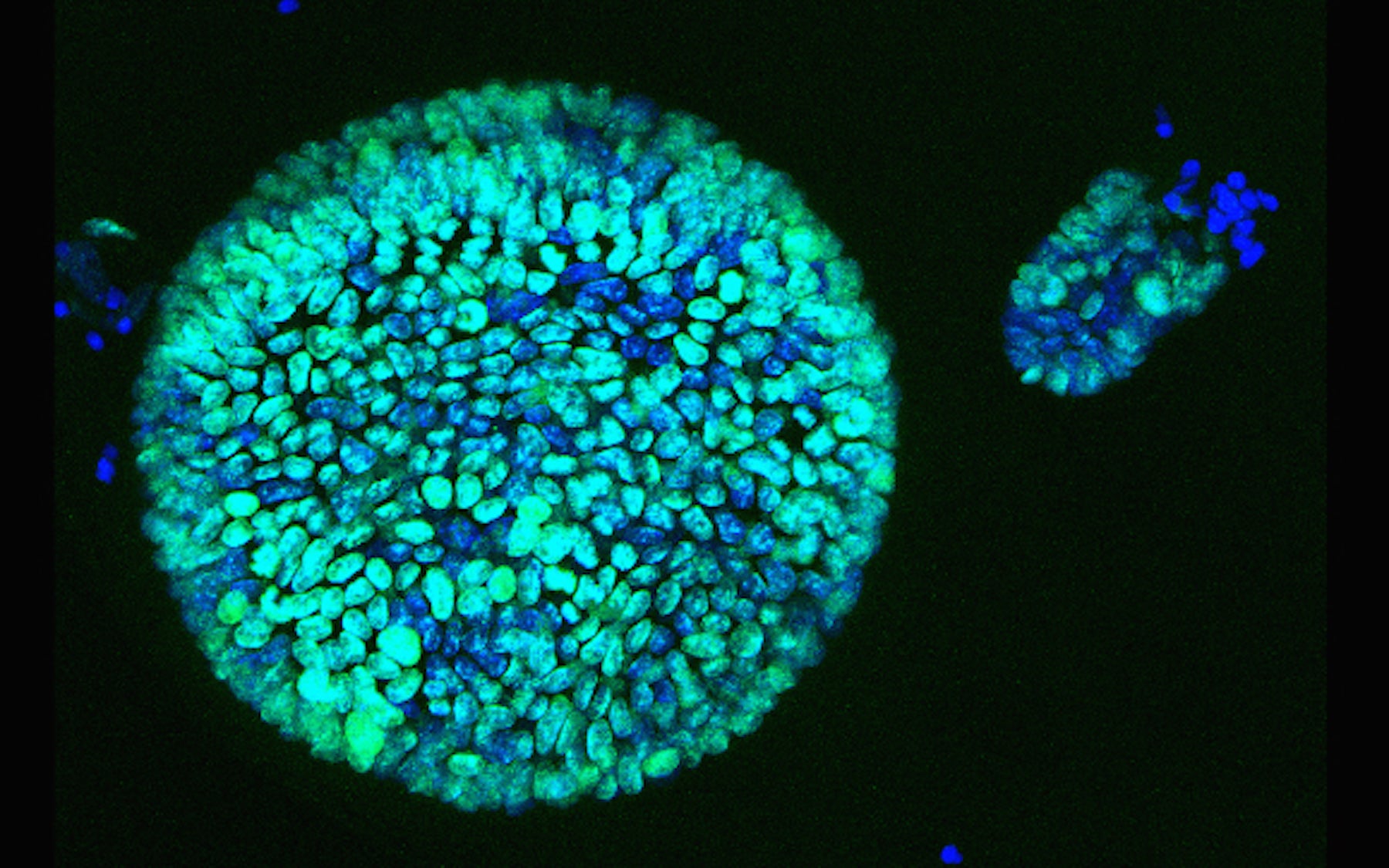
For Pancreatic Cancer Awareness Month this November, we explore the relationship between antioxidants and cancer cells, and how exploiting it could lead to better treatments for pancreatic cancer. We go into the lab to see the 3D pancreas “organoids” that the researchers used (see photo above), an innovation that allows them to study new therapies in ways never possible before. And we meet the some of the incredibly strong people who are fighting back through research after losing loved ones to this disease.
AA: And I’m Andrea Alfano.
BS: And this is Base Pairs, the podcast about the power of genetic information. We’re on our sixth episode now and somehow, we haven’t talked about cancer yet.
AA: I can’t believe it either. Scientists have been able to learn so much about cancer through genetic information.
BS: I mean, even in our daily lives we hear about this. Over the summer you probably saw ads for sunscreen that protects against some of the UV radiation that can damage your DNA and give rise to skin cancer. And then at the doctor we’re told about genetic tests for cancer genes, like the breast cancer gene BRCA1.
AA: Breast cancer is a great example of how genetic information has revolutionized the field of cancer research.
BS: It is. The survival rate for breast cancer has been increasing significantly over the past few decades. It’s up by roughly 25 percent, though that varies depending on what stage the doctors catch it at.
AA: That’s so important. Catching cancer at stage 1, before it’s had much of a chance to establish itself, and catching it at stage 4, when it’s already spread or metastasized throughout the body, are completely different situations.
BS: For breast cancer, genetic testing can give some families a heads-up. And mammograms—x-rays of the breasts—those have also really helped with early detection. While there’s controversy about how often to go for mammograms, that starts to seem less relevant once a woman or her doctor feels a lump.
AA: But cancer can also arise in places deep within the body, like in the pancreas—places where you can’t feel for lumps. Pancreatic cancer is incredibly difficult to catch early, partly because of this. And because it’s so hard to detect, the survival rate is really low. Only about 7 percent of patients live five years past diagnosis. Dannielle Engle, a postdoc researching pancreatic cancer in the lab of Dr. David Tuveson here at CSHL, knows this all too well.
DE: So I’m one of those people who has a very personal reason why I’m in the lab, and that’s because both my father and my uncle passed away from pancreatic cancer. Both my father and my uncle had stage four pancreatic cancer at the time of diagnosis.
BS: Stage four—that is not the stage you want to find cancer at.
AA: Not at all. It’s heartbreaking to hear how little there is to do for these patients.
DE: It just—it’s all over at that point. It’s so widely metastatic and they’re just also so sick at that point that even if we had effective therapy to give them it’s usually kind of hard to tolerate and so there’s very little that we can do besides makes these patients comfortable at the time of diagnosis. And I think that’s what a lot of people don’t realize, is that we have patients that only live for weeks after the time of diagnosis.
BS: Wow, so it’s kind of too late for chemo at that point, since by stage 4 the cancer has spread and usually can’t be brought under control by chemo.
AA: Basically, yeah. So there’s not a lot to do for these patients right now. But Dannie and her colleagues in Tuveson Lab are working on this problem. Some of them recently published a paper that describes a new potential avenue for treatment—one that only kills cancer, and leaves healthy cells unscathed. It has to do with antioxidants, which I think most people have heard of by now.
BS: Yeah, they destroy “free radicals” and prevent damage to DNA. Health food ads talk about them all the time.
[clip of POM Wonderful ad: https://www.youtube.com/watch?v= _HAk9zK8gTg]AA: Right. Free radicals are basically oxidants, which, as the names suggest, are the kinds of molecules that antioxidants neutralize. Antioxidants are definitely important. But there’s more to know than what the ads tell you…
[clip 0:04 – 0:33 from Consumer Reports video: http://www.consumerreports.org/cro/2013/03/antioxidants-more-is-not-always-better/index.htmAA: That clip is from a video by Consumer Reports. Scientists quoted in that video and across the scientific community say that our relationship with antioxidants is not so simple.
CC: I think like most of the general public, my view on antioxidants initially was that it’s good because it prevents aging, also prevents damage to our cells.
BS: That’s Christine Chio, a postdoc who works in the same lab as Dannielle and studies pancreatic cancer.
CC: The concept that I had initially was that you want to increase antioxidants to prevent cancer, to reduce progression. But as I slowly entered the field and I read the literature and I did some research myself, I realized that it’s quite the opposite—that while antioxidants might be good in certain settings in preventing aging, that’s also very much beneficial for the cancer cells.
BS: Antioxidants can benefit cancer cells. They definitely don’t mention that in the health food ads.
AA: Definitely not. But it’s really important to note that we are talking about situations where cancer already exists or is in the process of beginning — not in healthy people. These findings don’t apply to healthy people eating antioxidant-rich foods, so don’t throw away your blueberries or anything. Healthy cells in our bodies are constantly working to maintain a balance of oxidants and antioxidants that they need to survive.
BS: Still, it seems important.
AA: Agreed. This is one of the many examples of why talking to the public about science is so important.
BS: And the Tuveson Lab actually does quite a bit of it.
AA: They do! Sometimes it’s through large events like the Lustgarten Foundation’s annual pancreatic cancer awareness and fundraising walk, but sometimes it’s just sitting down with patients and their families over a meal—like when Dannielle recently sat down for lunch with the incredible Carol Whalen. After the immense tragedy of losing both her husband and daughter to pancreatic cancer, Carol immersed herself in scientific research on the disease, and has been meeting with Dannielle for years. As I sat eating my sandwich with them, Carol was furiously taking down notes and asking tons of questions.
CW: [crinkling of potato chip bags] Can you talk while eating? You have to multitask, Dannielle. There was an article in Newsweek about Dr. Chio, who found that antioxidants in the tumor or the tumor cell in the pancreas through working with organoids.
DE: Yes, Christine in our lab.
CW: What do you think of that?
DE: Well, I think what she’s found makes a lot of sense. And this is something that I think the clinic hasn’t quite caught up with the science.
AA: Christine is studying the effects that antioxidants have on cancer cells by looking into their DNA.
CC: I took a genetic approach in studying the effects of antioxidants. I study a gene called NRF2. So it is a master regulator of antioxidants in the body. In other words, when this protein is active, it leads to the production of antioxidants. And so the approach that I took was to try to genetically remove this gene, and by doing so, we can increase oxidants in the cells, and we ask “what are the effects to the cancer cells when we do this?”
BS: So they got rid of the gene for NRF2 in cancer cells. Meaning less antioxidants, and more oxidants.
AA: Right. In healthy, normal cells N-R-F-2 or “nerf” 2 serves as the guardian against stress to the cell. When the cell detects stress, like from UV radiation, for example, the NRF2 gene gets turned on. When certain foreign substances enter the cell, NRF2 gets turned on. It helps protect cells. Which sounds like a good thing, right? But then again…
CC: If you think about it from the perspective of a cancer cell, you would also realize that something like this would be very much desirable in a cancer cell to protect itself from the chemotherapeutics that we try to apply to the patient, for example. So there are many mechanisms through which NRF2 could, I guess, create chemo resistance.
BS: That makes so much sense. Cancer cells are like healthy cells in a lot of ways, but much more selfish and greedy. Of course they would take advantage of the good things that antioxidants can do for them.
AA: This blew my mind when I learned about it because it makes so much sense, just intuitively, and yet it’s so counter to the messages that we hear about antioxidants. This idea isn’t based solely in intuition, though. A growing body of evidence supports the idea that antioxidants can help cancer cells survive—a body of evidence that Christine and her colleagues are adding to.
CC: We found that when we increase oxidative stress [AA: That’s the kind of stress that antioxidants neutralize] through removing NRF2 there is a decrease in the activity of making proteins in cancer cells. So protein synthesis—or the making of proteins—is a very important procedure in any cell. This is true for normal cells, and this is MUCH more so for cancer cells. And when we do this [AA: when they remove NRF2, thereby LOWERING antioxidant levels] cancer cells don’t grow as well and some of them cannot survive, and therefore we think this might be a novel therapeutic approach that we can take.
BS: But don’t the healthy cells need antioxidants?
AA: Yes. But not as much as cancer cells do. Cancer cells are like normal cells on steroids, you might say
CC: The reason why we think this works is because when we applied the same strategy on normal cells we found that the effect is much, much less severe in the normal setting, and this is because the cancer cells have a much heavier dependence on the process of protein synthesis, or protein making.
[AA: Right, because they’re dividing so much.] Right. [AA: So every time they divide they need to make new little machines that keep the cell running, right?] That’s right. [AA: Ok, that makes sense.]BS: Hold on. So cancer cells divide a lot. That’s why they’re so problematic. And that means they have to make lots of new proteins, because these molecules do lots of jobs and every new cell needs its own set. And to make proteins, cells need antioxidants.
AA: Exactly. To divide more, a cell needs more antioxidants.
BS: Did they observe this effect in humans?
AA: Kind of. They haven’t tried this out in the clinic with real patients yet. But they did do tests on cells from real patients. This research is a new strategy, so they have to do a lot of testing in the lab before they can come up with a drug that’s ready for people to try. Instead, they used this super cool new system for studying the pancreas, both when it’s healthy and when there’s cancer.
CC: We have this beautiful platform of organoids which allows us to study normal cells, precancerous cells, and cancer cells.
AA: Organoids are basically balls of cells that they grow from small samples taken from either humans or mice. They put these samples in a dish that mimics the environment in the body, and this allows the cells to divide and grow in 3D, which is really important. This is what Carol Whalen really came to see during her latest lab visit with Dannielle. You’ll also hear Megan, a lab technician, and Carol’s husband, Mike Whalen.
DE: So when you look in the plate from the side—this is a well, and then what you’ll see is basically a little dome In this dome, you’ll see little tiny bubbles. And these bubbles are little spheres of cells that are the patient’s tumor cells growing as organoids. And so what we’re going to do is show you some normal and some malignant. And Megan, which one do we have on the microscope right now?
Meg: This is the normal.
DE: So I’ll just take a quick look to orient you guys and then what you can do is sit at this microscope and if you look in here, you’ll see a bunch of little bubbles. So feel free to sit down and take a look You see those? You see how they’re very neat and tidy? They’re all hollow, they’re all very thin-walled, and they all look very similar?
MW: They’re all different sizes.
DE: They’re all different sizes, but they’re all kind of the same shape, right? Take a look at these. These are kind of an example of our most aggressive, malignant-looking organoids. So when you look in there what do you see?
CW: Oh my gosh! Huge difference!
DE: And so tell me what differences you see.
CW: Well the size—well the number of large ones is lower, many more small, very small—some are teensy! And many are very dark throughout. Some are even different shades of gray throughout. And it looks like—I don’t know, it looks like some are kind of clumping together.
DE: Yes. So what is happening is—so a normal organoid is a hollow sphere with a single cell layer. These are solid. These are completely filled in.
AA: The head of this lab, David Tuveson, played a key role in developing this organoid system, which is used across a variety of cancers today—not just pancreatic cancer. Christine told me about why organoids are so powerful.
CC: So the development of this 3D culture is revolutionary because we now can maintain the genetic integrity of the cancer cells. And more importantly, this 3D culture supports the growth of normal cells. So now we have the ability to compare side-by-side normal cells and cancer cells.
BS: Scientists couldn’t get normal cells to grow in a flat Petri dish before? That’s incredible—being able to compare a drug’s effects on cancer cells with effects on normal cells is critical.
AA: It really has been revolutionary. This organoid system made it possible for Christine and her colleagues to do much more rigorous tests on potential new drugs that kill cancer—but not healthy cells—by lowering levels of antioxidants. They first tested the idea that lowering antioxidants could kill cancer by removing the NRF2 gene. But since we can’t exactly go around changing humans’ genes, for a variety of reasons, they set out to find a drug that has this effect.
CC: When we first found that this drug has an effect only in the cancer cells, I think that was the most exciting moment. But we think that this is still not the best drug combination because we can only achieve a modest improvement in survival. And the hope is that by studying these combinations in organoids and also in our mouse model, we can identify a better therapeutic combination.
BS: This is really just the beginning for this potential new therapy, then.
AA: Indeed. While it’s important to recognize that this work is still in an early stage, it’s an exciting start. And when dealing with a disease like this, where patients have such dismal options, new treatment ideas like this are pretty much the only source of hope. That’s also a big part of why Dannielle, is so passionate about spreading the word about pancreatic cancer research.
DE: I think one of the big problems with pancreatic cancer research is that we just need more people doing it. There’s a lot of work to be done and we need to encourage more young people to become scientists and to work on this problem, and I think that’s where participating in outreach and just talking about what I do has become very important to encouraging that in the future.
BS: She’s really like a missionary for pancreatic cancer research.
AA: Really. She’s an ambassador for the Lustgarten Foundation and is constantly looking for ways to spread the word about pancreatic cancer research—even talking with her mom on the phone has become an exercise in making her science accessible.
DE: I spend a lot of time practicing what I’m going to talk about with my mom, who’s not a scientist. She’s an accountant. So I spend a lot of time, like, on the phone explaining my research to her, so she understands very well what I do as a lay person. And so I think it’s important to talk about our research, just, like, to our friends and our family to make it more normal and accessible and they’ll be wanting to talk about with their friends so that we can eventually raise awareness of this disease and, you know, try and make a bigger difference.
AA: Dannielle and Christine are certainly doing their part, and so is everyone in their lab. I think it’s easy for people to imagine that scientists become detached from the people they’re supposed to serve, and in some cases that may be so. But not in this lab.
DE: My father passed away right when I started graduate school, so almost 10 years ago now. And it’s definitely very empowering. I do take my experimental failures I think a little bit harder because of it, but at the same time I have a unique drive and motivation to really fight pancreatic cancer because it’s personal with me. It’s really messed with my family and I’ve seen what it does to other families. And so I think as a scientist it’s important that we remember why we’re here and it’s definitely for those patients and families.
AA: The room where Dannielle and the rest of the team keep the organoids is what you would probably expect a lab to look like—sort of sterile and impersonal, with white walls and lots of equipment. But as Dannielle showed Carol around the room, the feeling that anything about that room is impersonal quickly washed away.
DE: So we have actually two incubators full right now. These are just more patients. And so right now, so you can see that some of them require very tender loving care, so Megan annotates those as being fragile. So you have to watch them very carefully to adjust to their needs. So something that Megan can attest to is that we’re here every day of the week. Sometimes our specimens come in at 8pm and we come in to isolate them, and we’re here until sometimes two in the morning.
Meg: Yeah [laughing] Takes a few hours.
CW: Just remember you’re doing it for us!
Meg: Yeah, I know!
DE: We’re here. We’re dedicated, and I think our success rate has to do with the fact that we are—we recognize how important it is.
Extras for Episode 6
Carol Whalen (white shirt in photo below), a tireless advocate for research on the disease that tragically claimed both her husband and daughter, stands alongside CSHL pancreatic cancer researchers at a fundraising walk held by the Lustarten Foundation. Dannielle Engle, the first researcher we meet in this episode, is standing directly to the right of Carol (Carol’s left), and Christine Chio, the second researcher we meet, is standing on the far right of the photo.
Pancreatic cancer researcher Christine Chio, a postdoc in the lab of David Tuveson, gives a quick overview of how she used 3D organoids to explore the relationship between antioxidants and pancreatic cancer cells in the video below.
Written by: Andrea Alfano, Content Developer/Communicator | webservices@cshl.edu | 516-367-8455