Michael James Lukey
Associate Professor
Cancer Center Assistant Director of Shared Resources
Ph.D., University of Oxford, U.K., 2010
lukey@cshl.edu | 516-367-5016
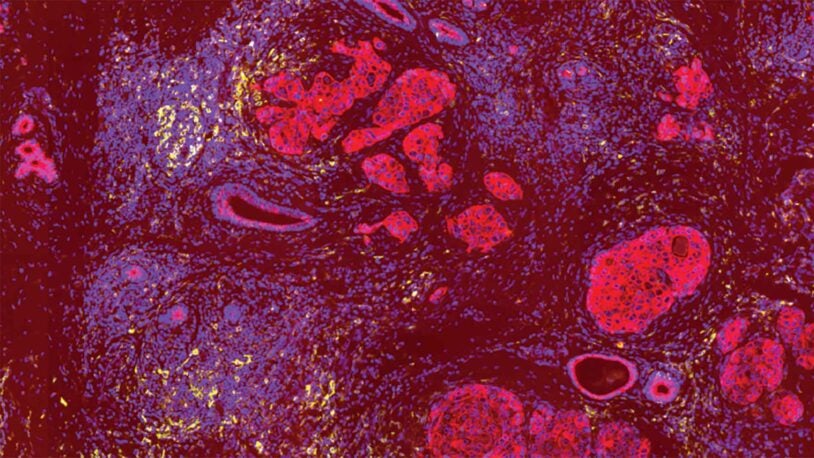
Faculty ProfileTumor growth depends upon cancer cells acquiring nutrients from their environment and using these molecules to fuel proliferation. My group studies the nature and regulation of metabolic adaptation during tumorigenesis and metastasis, with the intention of identifying metabolic vulnerabilities that can be targeted for cancer therapy.
Proliferative signals in mammalian cells drive biosynthetic programs that support cell growth and replication. In healthy cells this process is tightly regulated by growth factors, but in cancer cells oncogenic lesions can result in continuous signaling to the metabolic machinery. Oncogene-driven metabolic reprogramming supports tumorigenesis but renders cells sensitive to specific metabolic stresses, a phenomenon that is exploited for cancer therapy. Because the distribution of nutrients varies markedly between organs, cancer cells growing at different sites in the body – and in different regions of the tumor microenvironment—must employ a range of metabolic strategies to fuel their growth. We aim first to understand the nature and regulation of metabolic adaptations in the different stages of cancer, and then to develop therapeutic strategies that target resulting vulnerabilities. We are especially interested in the biochemical processes underlying nutrient sensing and metabolic/redox homeostasis, including regulation of the protein post-translational modification landscape by reactive metabolites. We are also exploring the reciprocal connections between tumor metabolism and host physiology, recognizing that metabolic therapies must be designed to synergize with, and not to antagonize, the anti-tumor immune response.
METAvivor Early Career Investigator Award Jan. 2020 – present
Breast Cancer Coalition of Rochester Research Award April 2017 – April 2018
Veronica Beard hosts CSHL–Penny’s Flight fundraiser
April 7, 2025
Fashion meets philanthropy as CSHL and Penny’s Flight Foundation partner with Veronica Beard to support groundbreaking research.
How breast cancer goes hungry
August 28, 2024
CSHL Assistant Professor Michael Lukey has identified a source of breast cancer’s backup food supplies—and a way to block access.
New hope in the fight against neurofibromatosis
March 18, 2024
A partnership between CSHL and the Penny’s Flight Foundation aims to find a cure for NF1, the world’s most common single-gene neurological disorder.
Test your breast cancer awareness
October 18, 2023
Awareness is key to prevention and potential future treatments. Take this quiz to find out about the latest in breast cancer research at CSHL.
President’s essay: Bringing bold visions to life
May 26, 2023
CSHL President & CEO Bruce Stillman sees the Laboratory as a global hub for scientific expertise and a powerful launchpad for early-career scientists.
A new, sustainable source for a promising cancer killer
March 23, 2023
This Malaysian jungle plant produces a chemical with remarkable anticancer properties. Now, CSHL scientists can synthesize that chemical in the lab.
How cancer metabolism could be key for new therapies
February 5, 2020
Assistant Professor Michael Lukey joins the CSHL faculty, studying metabolic reprogramming events in cancer.
All Publications
Glutamine metabolism is essential for coronavirus replication in host cells and in mice
9 Dec 2024 | Journal of Biological Chemistry | 301(1):108063
Greene, Kai; Choi, Annette; Yang, Nianhui; Chen, Matthew; Li, Ruizhi; Qiu, Yijian; Ezzatpour, Shahrzad; Rojas, Katherine; Shen, Jonathan; Wilson, Kristin; Katt, William; Aguilar, Hector; Lukey, Michael; Whittaker, Gary; Cerione, Richard;
The unique catalytic properties of PSAT1 mediate metabolic adaptation to glutamine blockade
Aug 2024 | Nature Metabolism | 6(8):1529-1548
Qiu, Yijian; Stamatatos, Olivia; Hu, Qingting; Ruiter Swain, Jed; Russo, Suzanne; Sann, Ava; Costa, Ana; Violante, Sara; Spector, David; Cross, Justin; Lukey, Michael;
Modular synthesis of functional libraries by accelerated SuFEx click chemistry
13 Mar 2024 | Chemical Science | 15(11):3879-3892
Homer, Joshua; Koelln, Rebecca; Barrow, Andrew; Gialelis, Timothy; Boiarska, Zlata; Steinohrt, Nikita; Lee, Erinna; Yang, Wen-Hsuan; Johnson, Robert; Chung, Taemoon; Habowski, Amber; Vishwakarma, Dharmendra; Bhunia, Debmalya; Avanzi, Charlotte; Moorhouse, Adam; Jackson, Mary; Tuveson, David; Lyons, Scott; Lukey, Michael; Fairlie, W; Haider, Shozeb; Steinmetz, Michel; Prota, Andrea; Moses, John;
Metabolic partitioning in the brain and its hijacking by glioblastoma
30 Aug 2023 | Genes & Development | 37:681-702
de Ruiter Swain, Jed; Michalopoulou, Evdokia; Noch, Evan; Lukey, Michael; Van Aelst, Linda;
Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia
11 Jul 2023 | Cell Metabolism | 35(7):1147-1162.e7
Ferrer, Miriam; Mourikis, Nicholas; Davidson, Emma; Kleeman, Sam; Zaccaria, Marta; Habel, Jill; Rubino, Rachel; Gao, Qing; Flint, Thomas; Young, Lisa; Connell, Claire; Lukey, Michael; Goncalves, Marcus; White, Eileen; Venkitaraman, Ashok; Janowitz, Tobias;
Inhibition of mitochondrial metabolism by (-)-jerantinine A: synthesis and biological studies in triple-negative breast cancer cells
26 Apr 2023 | RSC Medicinal Chemistry | 14(4):710-714
Gialelis, Timothy; Wang, Zifei; Homer, Joshua; Yang, Wen-Hsuan; Chung, Taemoon; Hu, Qingting; Smedley, Christopher; Pawar, Nitin; Upadhyay, Nitinkumar; Tuveson, David; Lyons, Scott; Lukey, Michael; Moses, John;
Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia
18 Feb 2023 | bioRxiv
Ferrer, Miriam; Mourikis, Nicholas; Davidson, Emma; Kleeman, Sam; Zaccaria, Marta; Habel, Jill; Rubino, Rachel; Flint, Thomas; Connell, Claire; Lukey, Michael; White, Eileen; Coll, Anthony; Venkitaraman, Ashok; Janowitz, Tobias;
Early Neutrophilia Marked by Aerobic Glycolysis Sustains Host Metabolism and Delays Cancer Cachexia
1 Feb 2022 | Cancers | 14(4):963
Petruzzelli, M; Ferrer, M; Schuijs, M; Kleeman, S; Mourikis, N; Hall, Z; Perera, D; Raghunathan, S; Vacca, M; Gaude, E; Lukey, M; Jodrell, D; Frezza, C; Wagner, E; Venkitaraman, A; Halim, T; Janowitz, T;
Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy
18 May 2021 | Trends in Cancer
Yang, Wen-Hsuan; Qiu, Yijian; Stamatatos, Olivia; Janowitz, Tobias; Lukey, Michael;
Lysine succinylation and SIRT5 couple nutritional status to glutamine catabolism.
19 Mar 2020 | Molecular and Cellular Oncology | 7(3):1735284
Lukey, M; Greene, K; Cerione, R;