David Tuveson
Professor
Roy J. Zuckerberg Professor of Cancer Research
Cancer Center Director
M.D., Ph.D., Johns Hopkins University, 1994
dtuveson@cshl.edu | 516-367-5246
Pancreatic cancer is an extremely lethal malignancy. On average, patients who are diagnosed with pancreatic cancer succumb to the disease within 6 months. Research is the only way to defeat pancreatic cancer. My lab is making progress toward finding a cure by detecting the disease earlier and designing novel therapeutic approaches.
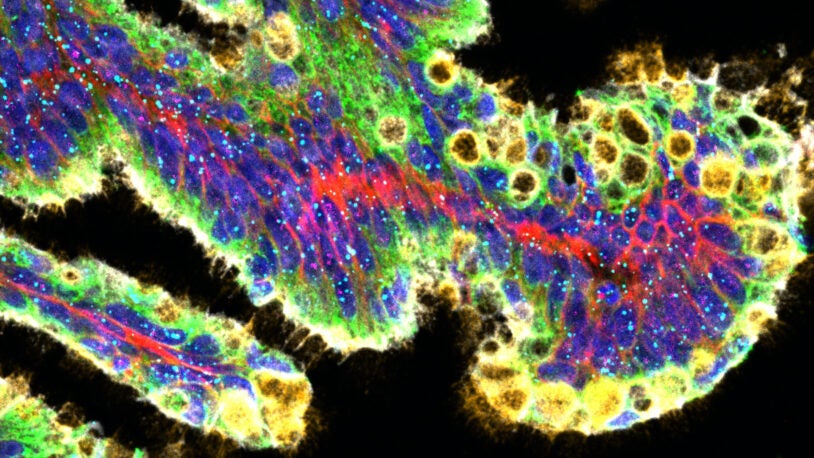
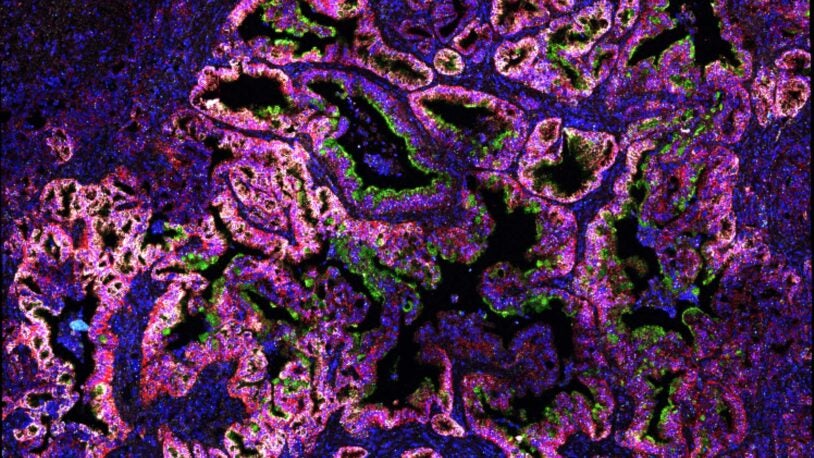
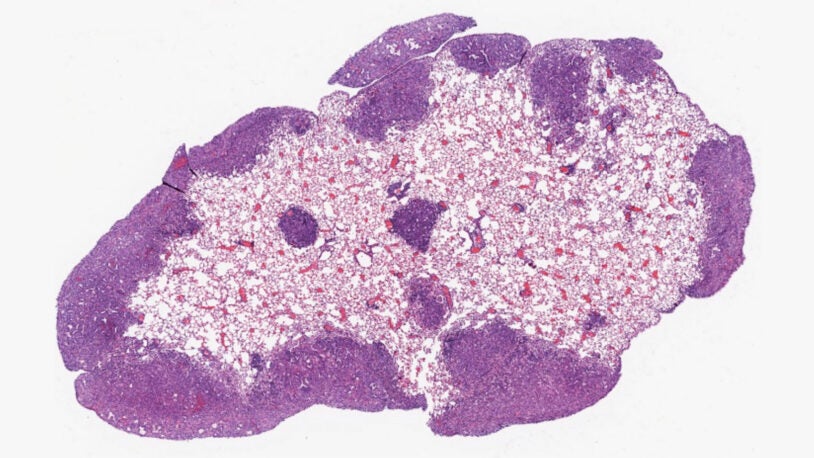
David Tuveson’s laboratory uses murine and human models of pancreatic cancer to explore the fundamental biology of malignancy and thereby identify new diagnostic and treatment strategies. The lab’s approaches run the gamut from designing new model systems of disease to developing new therapeutic and diagnostic approaches for rapid evaluation in preclinical and clinical settings. The lab’s studies make use of organoid cultures—three-dimensional cultures of normal or cancerous epithelia—as ex vivo models to probe cancer biology. Current projects in the lab explore changes in redox metabolism associated with pancreatic cancer tumorigenesis, dissect signaling by the Ras oncogene, discover new biomarkers of early pancreas cancer, and identify mechanisms of cross-talk between pancreatic cancer cells and the tumor stroma. Novel treatment approaches suggested by these studies are then tested by performing therapeutic experiments in mouse models. To dissect molecular changes associated with pancreatic tumorigenesis, the Tuveson lab has generated a large collection of human patient-derived organoid models. By measuring the therapeutic sensitivities of patient-derived organoids, the lab is working to identify novel strategies to treat patients as well as markers of therapeutic response. The Tuveson Laboratory maintains strong links to clinical research, and the ultimate goal is confirmation of preclinical findings in early-phase trials. Collectively, the lab’s bench-to-bedside approach is codified as the “Cancer Therapeutics Initiative,” and this initiative will provide these same approaches to the entire CSHL cancer community.
Dr. Tuveson serves as Director of the Cold Spring Harbor Laboratory Cancer Center and the Chief Scientist for the Lustgarten Foundation.
At the Lab: Catch me if you cancer
June 3, 2025
You’ve seen her on FOX and the cover of Newsday. Now, hear about the knowledge gaps that make her discovery so crucial and the noble goals driving it.
In pancreatic cancer, a race against time
April 2, 2025
By the time it’s diagnosed, it’s often too late. Now, Cold Spring Harbor Laboratory researchers have found a way to intercept the deadly disease.
Making headlines
September 11, 2024
Several Cold Spring Harbor Laboratory faculty members received national mainstream media attention in 2024.
Catching Cancer When It’s Curable
June 24, 2024
CSHL Trustee Bruce Ratner discusses his new book during a fireside chat with Professor and Cancer Center Director David Tuveson.
Tuveson elected to American Academy of Arts & Sciences
April 24, 2024
CSHL Cancer Center Director and Professor David Tuveson joins an elite membership roster whose historic ranks include names like Darwin and Einstein.
Nobel laureate honored at CSHL chemistry symposium
April 15, 2024
“The Future of Click Chemistry” brought together two-time Nobelist K. Barry Sharpless with his former apprentices John Moses and David Tuveson.
One Experiment: Pancreatic mucus
March 26, 2024
That’s not the Starship Enterprise burning up in space. It’s an up-close look at precancerous pancreatic lesions and the mucus they produce.
Pancreatic cancer lives on mucus
February 28, 2024
Mucus is not just snot. CSHL scientists have discovered some pancreatic cancer cells depend on it. The finding may open a new therapeutic avenue.
Pancreatic cancer hijacks a brain-building protein
February 14, 2024
CSHL researchers have found that EN-1, a protein involved in neurodevelopment, can help pancreatic cancer spread throughout the body.
CSHL announces AACR Cancer Centers Alliance
September 18, 2023
The new initiative, led by CSHL Professor David Tuveson, seeks to unite the nation’s NCI-designated cancer centers to help save more patients’ lives.
Selected Publications
Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade
7 Jun 2021 | Nature Communications | 12(1):3414
Nielsen, Sebastian; Strøbech, Jan; Horton, Edward; Jackstadt, Rene; Laitala, Anu; Bravo, Marina; Maltese, Giorgia; Jensen, Adina; Reuten, Raphael; Rafaeva, Maria; Karim, Saadia; Hwang, Chang-Il; Arnes, Luis; Tuveson, David; Sansom, Owen; Morton, Jennifer; Erler, Janine;
Oncogenic KRAS engages an RSK1/NF1 pathway to inhibit wild-type RAS signaling in pancreatic cancer.
25 May 2021 | Proceedings of the National Academy of Sciences of the United States of America | 118(21):e2016904118
Cheng, Derek; Oni, Tobiloba; Thalappillil, Jennifer; Park, Youngkyu; Ting, Hsiu-Chi; Alagesan, Brinda; Prasad, Nadia; Addison, Kenneth; Rivera, Keith; Pappin, Darryl; Van Aelst, Linda; Tuveson, David;
Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer
1 Apr 2021 | Clinical Cancer Research | 27(7):2023-2037
Steele, Nina; Biffi, Giulia; Kemp, Samantha; Zhang, Yaqing; Drouillard, Donovan; Syu, LiJyun; Hao, Yuan; Oni, Tobiloba; Brosnan, Erin; Elyada, Ela; Doshi, Abhishek; Hansma, Christa; Espinoza, Carlos; Abbas, Ahmed; The, Stephanie; Irizarry-Negron, Valerie; Halbrook, Christopher; Franks, Nicole; Hoffman, Megan; Brown, Kristee; Carpenter, Eileen; Nwosu, Zeribe; Johnson, Craig; Lima, Fatima; Anderson, Michelle; Park, Youngkyu; Crawford, Howard; Lyssiotis, Costas; Frankel, Timothy; Rao, Arvind; Bednar, Filip; Dlugosz, Andrzej; Preall, Jonathan; Tuveson, David; Allen, Benjamin; Pasca di Magliano, Marina;
Fighting the Sixth Decade of the Cancer War with Better Cancer Models.
Apr 2021 | Cancer Discovery | 11(4):801-804
Tuveson, David;
Diversity and Biology of Cancer-Associated Fibroblasts.
1 Jan 2021 | Physiological Reviews | 101(1):147-176
Biffi, Giulia; Tuveson, David;
All Publications
The hunt for common tumor antigens
8 May 2025 | Science | 388(6747):592-593
Tuveson, David;
Macrophages and fibroblasts as regulators of the immune response in pancreatic cancer
22 Apr 2025 | Nature Immunology
Shakiba, Mojdeh; Tuveson, David;
Road Map to Defeat Pancreatic Cancer
Apr 2025 | Annual Review of Cancer Biology | 9(1):1-20
Ferguson, L; Tuveson, David;
FGFR2 Abrogation Intercepts Pancreatic Ductal Adenocarcinoma Development
2 Apr 2025 | Cancer Research
Tonelli, Claudia; Deschênes, Astrid; Gaeth, Victoria; Jensen, Amanda; Vithlani, Nandan; Yao, Melissa; Zhao, Zhen; Park, Youngkyu; Tuveson, David;
GATA6 Amplification Is Associated With Improved Survival in TP53-Mutated Unresectable Pancreatic Cancer
1 Apr 2025 | Pancreas | 54(4):e303-e309
Zhou, Edward; Yang, Jung-In; Habowski, Amber; Deschênes, Astrid; Belleau, Pascal; Ha, Taehoon; Tzanavaris, Chris; Boyd, Jeff; Hollweg, Christopher; Zhu, Xinhua; Tuveson, David; King, Daniel;