Lingbo Zhang
Assistant Professor
Cancer Center Member
Ph.D., Joint program of Massachusetts Institute of Technology and National University of Singapore, 2013
lbzhang@cshl.edu | 516-367-5414
Faculty ProfileThe research in the Zhang laboratory centers on normal and malignant stem and progenitor cells in the hematopoietic system and decodes the role of metabolites in the tumor microenvironment, including nutrients and neurotransmitters, and their genetic effectors in regulating hematologic malignancies. The ultimate goal is to understand how environmental signals such as dietary and neuronal activities regulate stem and progenitor cell development and cancers.
Our research focuses on decoding the role of metabolites and their genetic effectors in the tumor microenvironment of hematologic malignancies. We utilize a combination of functional genomics, metabolomics, circuit mapping, and optogenetics approaches to systematically uncover critical dietary and neuronal activities that regulate stem and progenitor cell development and identify key drug targets to treat hematologic malignancies.
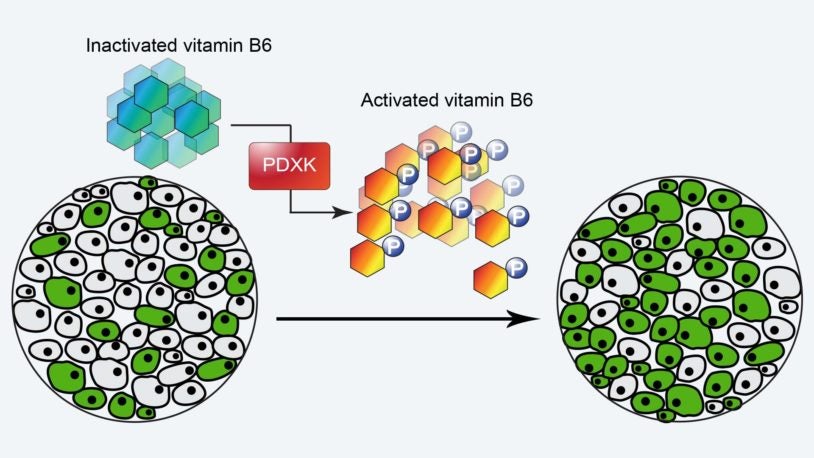
Together, the Zhang laboratory recently uncovered a series of critical metabolites in the tumor microenvironment, including acetylcholine and pyridoxal, and their genetic effectors as novel regulators of hematologic malignancies. We identified the cholinergic receptor muscarinic 4 (CHRM4) as a novel regulator of early erythroid progenitor self-renewal and a therapeutic target for myelodysplastic syndromes (MDS). Our research uncovered the hematopoietic arc as a novel neuronal activity based regulatory mechanism of hematopoietic stem and progenitor cell self-renewal. We also identified the vitamin B6 pathway as a nutritional and metabolic dependency in acute myeloid leukemia (AML) that coordinates nucleotides and putrescine metabolism specifically required for leukemia maintenance. Our research uncovered the vitamin B6 pathway as a pharmacologically actionable target for the treatment of leukemia with minimal myelosuppression effect. Through collaboration with medicinal chemists at our spin-off company, we are building pharmacological approaches at the pharmaceutical industry level to target these novel regulators and translating our discoveries into first-in-class therapeutics, and our findings will help treat devastating hematologic malignancies, including refractory anemia, myelodysplasia and leukemia.
National Institutes of Health/National Cancer Institute MERIT Award
Congressionally Directed Medical Research Programs Idea Development Award
National Institutes of Health Research Evaluation and Commercialization Hub Awards
Edward P. Evans Foundation EvansMDS Young Investigator Award
Chinese Government Award for Outstanding Self-finance Students Abroad
Tsinghua University Outstanding Master Degree Thesis Award
CSHL receives $100K for leukemia research
February 24, 2025
The Don Monti Memorial Research Foundation’s donation supports the Zhang lab’s ongoing research on several variants of the deadly blood cancer.
President’s essay: The continuous cycle of discovery
May 30, 2024
CSHL President & CEO Bruce Stillman discusses our institution’s societal impacts and global connections as forces for further scientific progress.
How diet may impact cancer and possible treatments
February 1, 2024
Researchers at the CSHL Cancer Center study the links between disease and nutrition in hopes of uncovering new treatment and prevention strategies.
Bite into this diet and disease quiz
July 5, 2023
Test your knowledge of how diet and nutrition affect health and disease with this short quiz.
Lingbo Zhang wins National Institutes of Health MERIT Award
June 15, 2023
The highly prestigious award will support Zhang’s research on the role of nutrients and other environmental factors in blood cancer development.
President’s essay: Bringing bold visions to life
May 26, 2023
CSHL President & CEO Bruce Stillman sees the Laboratory as a global hub for scientific expertise and a powerful launchpad for early-career scientists.
Event: Canceled Cocktails & Chromosomes
February 18, 2020
As a precautionary measure to ensure the safety of our employees and visitors during the current COVID-19 outbreak, Cold Spring Harbor Laboratory (CSHL) is canceling and/or postponing events for public audiences.
Vitamin B6, leukemia’s deadly addiction
January 13, 2020
Acute Myeloid Leukemia is addicted to vitamin B6. Now that researchers know this, they can pursue new treatment options for the deadly blood cancer.
Vitamin B6, leukemia’s deadly addiction
January 13, 2020
Acute Myeloid Leukemia is addicted to vitamin B6. Now that researchers know this, they can pursue new treatment options for the deadly blood cancer.
Discovery could improve MDS cancer treatment
September 25, 2019
Researchers have discovered a promising new target for treating myelodysplastic syndrome (MDS), a common and lethal blood cancer.
Nature Chemical Biology – Hooked on vitamins
World Pharma News – Vitamin B6, leukemia’s deadly addiction
Medical News Today – Leukemia’s unexpected link to vitamin B-6
Drug Target Review – A novel target revealed for myelodysplastic syndrome
BioSpace – Newly discovered target overcomes drug resistance in lethal blood cancer
Bioengineer – Discovery could improve MDS cancer treatment
LI Bioscience Hub Backs 10 In New Funding Round
Scientists identify potential drug target for treatment-resistant anemias
Selected Publications
Metabolism in acute myeloid leukemia: mechanistic insights and therapeutic targets
22 Dec 2022 | Blood
Mishra, Sushanta; Millman, Scott; Zhang, Lingbo;
Targeting low-risk myelodysplastic syndrome with novel therapeutic strategies
Jul 2021 | Trends in Molecular Medicine
Trivedi, Gaurang; Inoue, Daichi; Zhang, Lingbo;
Vitamin B6 Addiction in Acute Myeloid Leukemia
13 Jan 2020 | Cancer Cell | 37(1):71-84
Chen, C; Li, B; Millman, S; Chen, C; Li, X; Morris, J; Mayle, A; Ho, Y; Loizou, E; Liu, H; Qin, W; Shah, H; Violante, S; Cross, J; Lowe, S; Zhang, L;
Muscarinic acetylcholine receptor regulates self-renewal of early erythroid progenitors
2019 | Science Translational Medicine | 11(511)
Trivedi, Gaurang; Inoue, Daichi; Chen, Cynthia; Bitner, Lillian; Chung, Young; Taylor, Justin; Gönen, Mithat; Wess, Jürgen; Abdel-Wahab, Omar; Zhang, Lingbo;
ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors
4 Jul 2013 | Nature | 499(7456):92-6
Zhang, L; Prak, L; Rayon-Estrada, V; Thiru, P; Flygare, J; Lim, B; Lodish, H;
All Publications
MicroRNAs in erythroid and megakaryocytic differentiation and megakaryocyte-erythroid progenitor lineage commitment
Nov 2012 | Leukemia | 26(11):2310-6
Zhang, L; Sankaran, V; Lodish, H;
From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications
8 Dec 2011 | Blood | 118(24):6258-68
Hattangadi, S; Wong, P; Zhang, L; Flygare, J; Lodish, H;
miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1
15 Jan 2011 | Genes & Development | 25(2):119-24
Zhang, L; Flygare, J; Wong, P; Lim, B; Lodish, H;