Nicholas Tonks
Professor
Caryl Boies Professor of Cancer Research
Cancer Center Associate Director of Shared Resources
Ph.D., University of Dundee, 1985
tonks@cshl.edu | 516-367-8846
Faculty ProfileCells must constantly react to what is happening around them, adapting to changes in neighboring cells or the environment. I study the signals that cells use to exchange information with their surroundings. Our group is finding drugs that target these signals and thus can treat diabetes, obesity, cancer, and autism spectrum disorders.
Nicholas Tonks and colleagues study a family of enzymes called protein tyrosine phosphatases, or PTPs, which remove phosphate groups from proteins and other signaling molecules, such as lipids, in cells. Disruption of PTP function is a cause of major human diseases, and several of the PTPs are potential therapeutic targets for such diseases. Tonks’ group seeks to fully characterize the PTP family, understanding how PTP activity is controlled and how PTPs modify signaling pathways. In addition, they are working to determine how those pathways are abrogated in serious illnesses, including cancer, diabetes, and Parkinson’s disease. The overall goal is to identify new targets and strategies for therapeutic intervention in human disease. Tonks and colleagues have defined new roles for PTPs in regulating signaling events in breast cancer, identifying three PTPs as novel potential tumor suppressors. They have characterized the regulation of PTP1B by reversible oxidation, demonstrating that it is regulated by covalent modification of the active site by hydrogen sulfide (H2S) under conditions of ER stress that are linked to protein-folding-related pathologies, such as Parkinson’s and Alzheimer’s. In addition, they have generated recombinant antibodies that selectively recognize the oxidized conformation of PTP1B; these antibodies display the ability to promote insulin signaling in cells and suggest novel approaches to therapy for diabetes. Finally, they have also discovered a novel mechanism for allosteric regulation of PTP1B activity, offering the possibility of developing small-molecule drugs that could inhibit the phosphatase and thereby modulate signaling by insulin and the oncoprotein tyrosine kinase HER2, potentially offering new ways to treat insulin resistance in type-2 diabetes and breast cancer.
Women’s health quiz
March 19, 2024
CSHL research has yielded insights into a number of women’s health topics, from menopause to breast cancer. Take this quiz to see how far we’ve come.
A quiz for the ages
January 29, 2024
Want to know the secret to a long life? So do CSHL scientists. Take this short quiz to see what they’ve found out about aging and longevity.
New antibody could target breast cancers
October 30, 2023
CSHL Professor Nicholas Tonks’ team has discovered a new way to target PTPRD, an enzyme that may help some breast cancers spread.
President’s essay: Bringing bold visions to life
May 26, 2023
CSHL President & CEO Bruce Stillman sees the Laboratory as a global hub for scientific expertise and a powerful launchpad for early-career scientists.
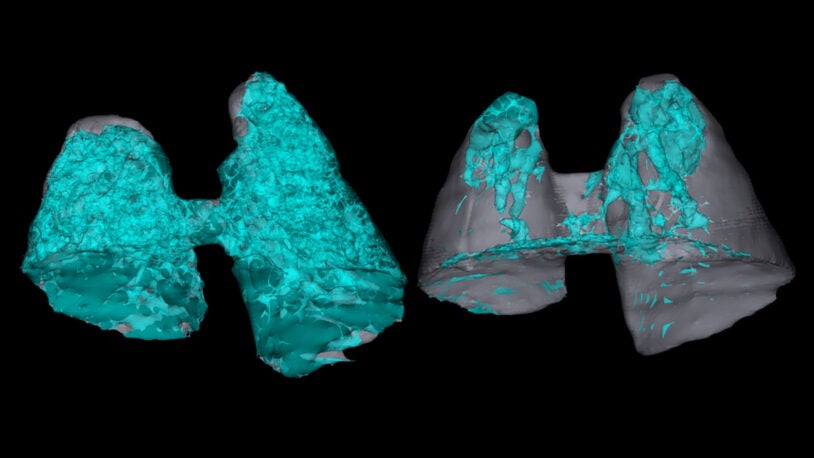
Disarming the immune system’s lethal lung response
September 23, 2022
CSHL researchers found a drug that prevents lethal lung inflammation in mice, which may lead to better treatments for severe inflammatory conditions.
Copper, cancer, and COVID-19: The story of an idea, a meeting, and a paper
March 4, 2022
The Copper Cancer Consortium started as a new treatment concept and is now a published paper. Read the story of Banbury's last meeting before COVID.
Research matters
June 8, 2021
Innovative research and educational activities never stopped during the COVID-19 pandemic.
Nicholas Tonks named 2019 AAAS Fellow
November 26, 2019
CSHL Professor Nicholas Tonks is being honored as a 2019 AAAS Fellow for his work on signal transduction and the discovery of PTP.
Historic building—groundbreaking science
October 29, 2019
The Demerec building has been monumental in scientific history. Now, a $75 million renovation of this celebrated labspace will define CSHL’s future.
Possible treatment for Wilson’s disease unexpectedly identified
July 31, 2018
Dr. Nicholas Tonks describes how the serendipity inherent to basic research played its part in discovering a potential treatment for Wilson's disease.
All Publications
PTPN23-dependent ESCRT machinery functions as a cell death checkpoint
28 Nov 2024 | Nature Communications | 15(1):10364
Song, Dongyan; Cen, Yuxin; Qian, Zhe; Wu, Xiaoli; Rivera, Keith; Wee, Tse-Luen; Demerdash, Osama; Chang, Kenneth; Pappin, Darryl; Vakoc, Christopher; Tonks, Nicholas;
Famotidine increases cellular phospho-tyrosine levels
28 Sep 2024 | Biochemical and Biophysical Research Communications | 734:150763
Zubiete-Franco, Imanol; Tonks, Nicholas;
Manipulating PTPRD function with ectodomain antibodies
5 Sep 2023 | Genes & Development
Qian, Zhe; Song, Dongyan; Ipsaro, Jonathan; Bautista, Carmelita; Joshua-Tor, Leemor; Yeh, Johannes; Tonks, Nicholas;
Protein Tyrosine Phosphatases: Mighty oaks from little acorns grow
Apr 2023 | IUBMB Life | 75(4):337-352
Tonks, Nicholas;
Coupling substrate-trapping with proximity-labeling to identify protein tyrosine phosphatase PTP1B signaling networks
3 Mar 2023 | Journal of Biological Chemistry | :104582
Bonham, ChristopherA; Mandati, Vinay; Singh, RakeshK; Pappin, DarrylJ; Tonks, NicholasK;
PTP1B inhibitors protect against acute lung injury and regulate CXCR4 signaling in neutrophils
22 Jul 2022 | JCI insight | 7(14):e158199
Song, Dongyan; Adrover, Jose; Felice, Christy; Christensen, Lisa; He, Xue-Yan; Merrill, Joseph; Wilkinson, John; Janowitz, Tobias; Lyons, Scott; Egeblad, Mikala; Tonks, Nicholas;
PTP1B is an intracellular checkpoint that limits T cell and CAR T cell anti-tumor immunity.
18 Nov 2021 | Cancer Discovery
Wiede, Florian; Lu, Kun-Hui; Du, Xin; Zeissig, Mara; Xu, Rachel; Goh, Pei; Xirouchaki, Chrysovalantou; Hogarth, Samuel; Greatorex, Spencer; Sek, Kevin; Daly, Roger; Beavis, Paul; Darcy, Phillip; Tonks, Nicholas; Tiganis, Tony;
Connecting copper and cancer: from transition metal signalling to metalloplasia
11 Nov 2021 | Nature Reviews Cancer
Ge, Eva; Bush, Ashley; Casini, Angela; Cobine, Paul; Cross, Justin; DeNicola, Gina; Dou, Q; Franz, Katherine; Gohil, Vishal; Gupta, Sanjeev; Kaler, Stephen; Lutsenko, Svetlana; Mittal, Vivek; Petris, Michael; Polishchuk, Roman; Ralle, Martina; Schilsky, Michael; Tonks, Nicholas; Vahdat, Linda; Van Aelst, Linda; Xi, Dan; Yuan, Peng; Brady, Donita; Chang, Christopher;
Author Correction: Mito-oncology agent: fermented extract suppresses the Warburg effect, restores oxidative mitochondrial activity, and inhibits in vivo tumor growth.
29 Jan 2021 | Scientific Reports | 11(1):3036
Bencze, Gyula; Bencze, Szilvia; Rivera, Keith; Watson, James; Hidvegi, Mate; Orfi, Laszlo; Tonks, Nicholas; Pappin, Darryl;
Mito-oncology agent: fermented extract suppresses the Warburg effect, restores oxidative mitochondrial activity, and inhibits in vivo tumor growth
25 Aug 2020 | Scientific Reports | 10(1):14174
Bencze, G; Bencze, S; Rivera, K; Watson, J; Orfi, L; Tonks, N; Pappin, D;