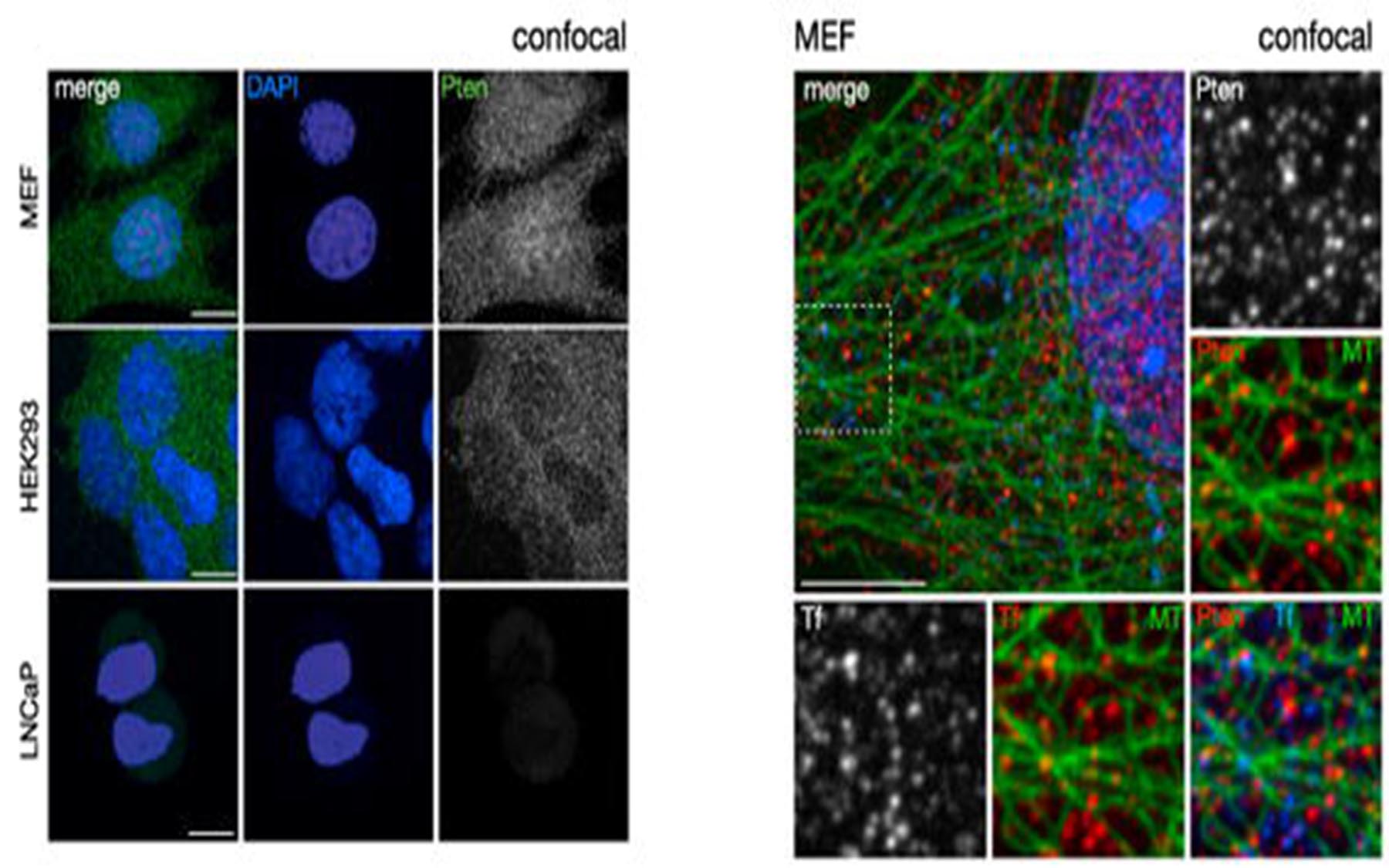
The gene called PTEN is one of the most important of the body’s natural tumor suppressors.
Cold Spring Harbor, NY — The gene called PTEN is one of the most important of the body’s natural tumor suppressors. When the gene is mutated or missing, as it is often observed to be in a host of cancers, growth signals affecting cells can get stuck in the “on” position, enabling cells to proliferate out of control.
Today, scientists at Cold Spring Harbor Laboratory (CSHL) publish new evidence explaining precisely how the protein encoded by PTEN (called PTEN) works—specifically, how it is recruited to particular locations in our cells where pro-growth signals need to be shut off.
The new evidence, assembled by a team led by CSHL Associate Professor Lloyd Trotman, contradicts a long-held assumption about PTEN function, and could help scientists design more effective drugs to counteract cancer’s hallmark trait, uncontrolled cellular growth.
“A whole generation of cancer investigators, including me, has been taught that PTEN performs its crucial role at the plasma membrane, which is what separates the inside of cells from the outside environment” Trotman explains. The exterior surface of the membrane is dotted with receptor molecules—switches which growth factors can flip on from the outside to transmit growth cues into the cell’s interior.
Normally, these switches are off, and no signals are transmitted. Every once in a while, however, a pro-growth-hormone molecule docks at a receptor on the surface, setting off a cascade of biochemical events inside the cell. “With respect to growth, the brakes are normally on,” says Trotman. In the cell membrane, fatty molecules are decorated with the equivalent of traffic signals, and “in the default mode, the light is red.” But when a growth factor switches on a receptor, special enzymes change the light embedded in the membrane from red to green. Then, after the signal is transmitted further into the cell’s interior, it is time to switch the signal back to red. This is the job of the PTEN protein.
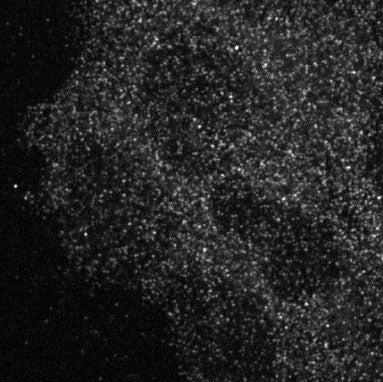
Since the discovery of these fundamental signaling mechanisms about 15 years ago, scientists have assumed that PTEN performs this crucial task at or near the interior surface of the cell membrane. But how does it get there? And where does it comes from? When Trotman and colleagues imaged the interior of ordinary cells, they found PTEN proteins everywhere—not just near the membrane, but virtually everywhere throughout the three-dimensional space of the cells (see image above). Prevailing theory did not consider this a problem: “it had PTEN bouncing around, all over the cell, and abundant enough so that it was always available near the membrane,” to switch a green growth signal back to red.
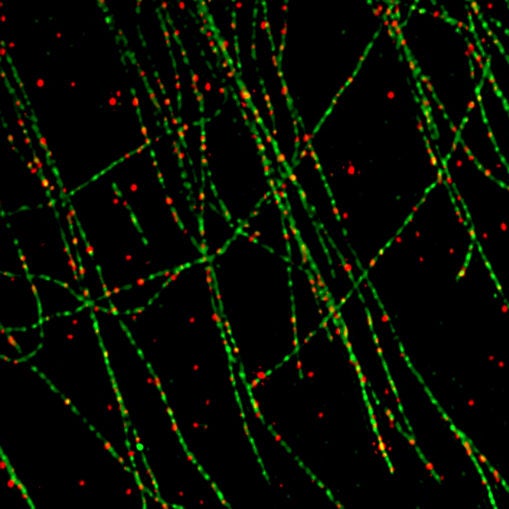
“What we found was that PTEN is not, as the theory suggests, literally bouncing off the walls of the cell in random fashion,” Trotman says. “Using super-resolution microscopy, the technology that was awarded last year’s Nobel Prize in Chemistry, we were able to discover an organizing principle at work.” The team was amazed to find a consistent association, throughout cells: the location of PTEN proteins closely coincided with the presence of tiny highways called microtubules that crisscross throughout every cell.
PTEN proteins travel along microtubule highways to where they are needed. But how do they know when and where, precisely? In a paper appearing today in Molecular Cell, Trotman’s team explains that green signals ready to be turned to red are literally pinched off from the cell membrane in tiny bubble-like structures called vesicles. This process is called endocytosis. The vesicles are coated with a protein called clathrin, which helps the vesicles to take spherical shape. Later, the coating is dissolved, exposing the naked green signal as well as a magnet-like signal to attract PTEN. This is the moment, according to Trotman’s team, when PTEN is recruited. PTEN then binds the vesicle bearing the green signal and performs its essential service: it removes a phosphate group from the signal (a process called de-phosphorylation), turning the signal back to red.
Trotman’s group demonstrates how PTEN’s structure is precisely evolved to do the job. One domain of the protein is structured to bind to the magnet; its other domain catalyzes the phosphate-removing reaction. “It is quite an elegant mechanism,” Trotman says. “It is highly efficient. And in view of PTEN’s critical role as a tumor suppressor, it’s also important that the process we uncovered is a controlled one, not random as was previously believed.”
Many observed facts “fall into place” with the new explanation of how PTEN works. “Now we understand this fundamental process. We understand that taking growth signals inside the cell via vesicles, is directly related to the process of turning them off. The vesicle is essentially packaging. Once the growth signal gets packaged, it’s being readied to be shut off.”
Written by: Peter Tarr, Senior Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
The research described in this release was funded by the American Cancer Society; the Pershing Square Sohn Foundation; the Department of Defense; the National Institutes of Health; and the Robertson Research Fund of CSHL.
Citation
“PTEN functions by recruitment to cytoplasmic vesicles” appears online ahead of print April 9, 2015 in Molecular Cell. The authors are: Adam Naguib, Gyula Bencze, Hyejin Cho, Ante Tocij, Elad Elakayam, Christopher R. Faehnle, Nadia Jaber, Christopher P. Pratt, Muhan Chen, Wei-xing Zong, Michael S. Marks, Leemor Joshua-Tor, Darryl J. Pappin and Lloyd C. Trotman. The paper can be obtained at: http://www.sciencedirect.com/science/journal/aip/10972765
Principal Investigator

Lloyd Trotman
Professor
Cancer Center Deputy Director of Education
Ph.D., University of Zurich, 2001