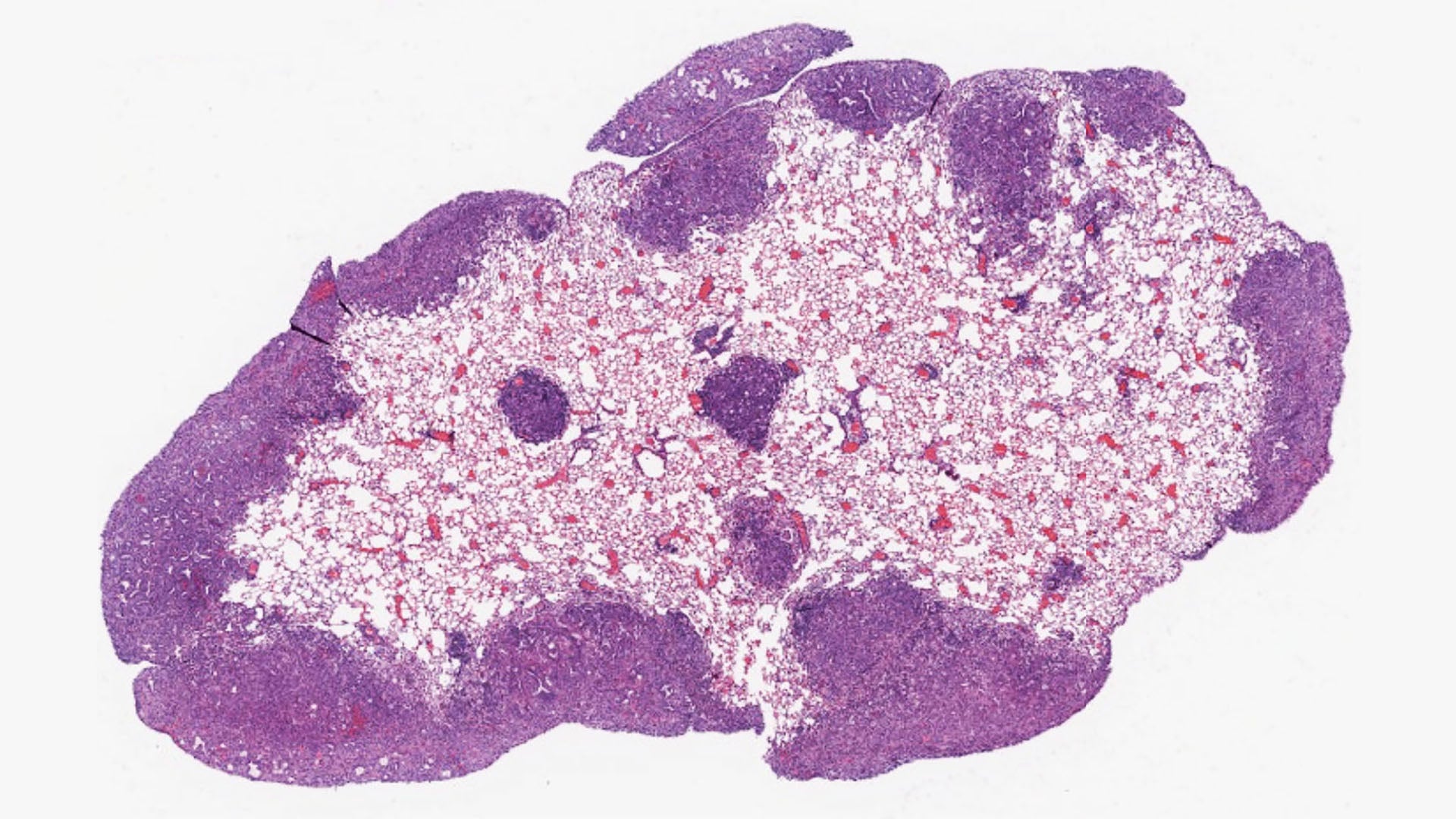
Scientists at Cold Spring Harbor Laboratory (CSHL) and the University of California, Davis have reached a new breakthrough in pancreatic cancer research—eight years in the making. It could help slow the disease’s deadly spread.
In 2017, as a postdoc in CSHL’s Tuveson lab, Chang-il Hwang and collaborators from the Vakoc lab uncovered a protein essential for jumpstarting metastasis in pancreatic ductal adenocarcinoma (PDAC). Now an assistant professor at UC Davis, Hwang recently reunited with CSHL Professors David Tuveson and Christopher Vakoc. The trio once again set their sights on PDAC. The disease is known for its aggressiveness. It often spreads to other organs like wildfire, wreaking havoc in its path.
“Metastatic spread is one of the reasons pancreatic cancer is such a deadly disease,” Vakoc says. “Our study led by Dr. Hwang has opened the door to new insights we might one day use to combat aggressive PDAC.”
The team found that late-stage PDAC hijacks a protein called Engrailed-1 (EN-1) to evade the body’s natural cancer defenses. EN-1 is a type of protein known as a transcription factor. These proteins control the timing and duration of gene expression. This particular transcription factor is required to form major areas of the brain.
“EN-1 is known to play a role in neurodevelopment,” Hwang explains. “In the pancreas, it’s not normally expressed. But in the later stages of pancreatic cancer, it gets overly expressed and makes the cancer more metastatic.” That means a faster, deadlier spread. But what if EN-1 could be targeted in cancer? Hwang, Tuveson, and Vakoc sought to find out.
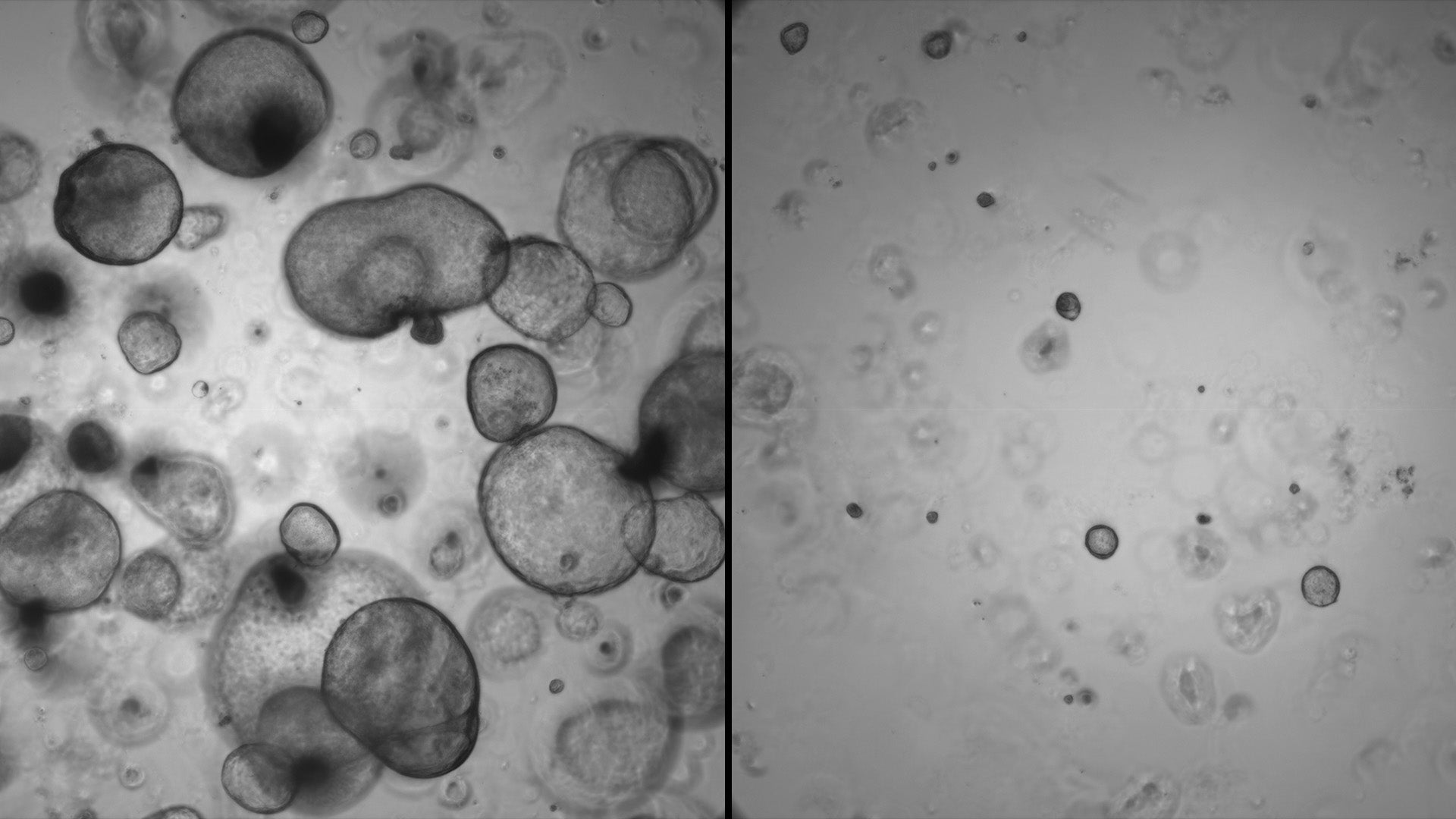
The team used organoids—mini versions of tumors—to identify the role of over-expressed EN-1 in PDAC. They found that higher levels of the aberrant protein blocked genes associated with natural cell death. When EN-1 expression was curtailed, the genes it targets were able to do their job, promoting healthy cell survival.
“Without EN-1, cancer progression slows,” Hwang says. “At the moment, it’s hard to target transcription factors with drugs. But in the future, it may be possible to disrupt the kind of interactions we see with mutated EN-1 in PDAC.”
Pancreatic cancer remains the third-leading cause of cancer-related deaths in the U.S. Hwang plans to continue collaborating with CSHL in hopes that his team’s work may lead to better treatments.
“We know certain types of PDAC are dependent on EN-1,” Hwang says. “If we can develop a way to test for it, we can create more personalized therapeutics and treatment strategies for patients. We’re looking forward to heading in that direction.”
Written by: Nick Wurm, Communications Specialist | wurm@cshl.edu | 516-367-5940
Funding
National Institute of Environmental Health Sciences, National Cancer Institute, UC Davis Comprehensive Cancer Center, National Research Foundation of Korea, Brain Korea 21, Lustgarten Foundation, Cold Spring Harbor Laboratory Association, The V Foundation for Cancer Research, Thompson Foundation for Autism & Neurodevelopment, National Institutes of Health, Simons Foundation, Pancreatic Cancer Detection Consortium
Citation
Xu, J., et al., “Engrailed-1 Promotes Pancreatic Cancer Metastasis”, Advanced Science, December 18, 2023 DOI: 10.1002/advs.202308537