A new tool to select the treatments most likely to work in specific patients
Cold Spring Harbor, NY — Patient-derived organoids, hollow spheres of cells cultured from tumors, can quickly and accurately predict how patients with pancreatic cancer respond to a variety of treatments, facilitating a precision-medicine approach to the deadly disease. The ability to use organoids as a treatment tool was investigated by an international team of researchers led by Dr. David Tuveson, M.D., Ph.D., Cold Spring Harbor Laboratory (CSHL) Professor and Chief Scientist for The Lustgarten Foundation.
“We’ve identified an approach to prioritize treatment strategies for pancreas cancer patients, with the goal of giving them the best shot at survival and the best shot at a good quality of life,” says Dr. Hervé Tiriac, a researcher in Tuveson’s lab and first author of the paper published today in Cancer Discovery.
With only 8 percent of patients surviving 5 years beyond their diagnosis, pancreatic cancer is one of the deadliest cancer types. Currently, surgical removal of the cancerous tissue is the only effective treatment, but because the disease progresses so quickly, only 15 percent of patients are eligible for the procedure. Surgery-ineligible patients can be treated with drugs or chemotherapy, but patient response is highly varied and there is no good method to determine which treatment is best for any given patient.
For several years, Tuveson has been honing organoid technology to improve research into the disease. Aside from taking only as little as six weeks to grow, a major advantage of organoids is that they can be derived from patients with even very advanced pancreatic cancer, using tiny biopsies.
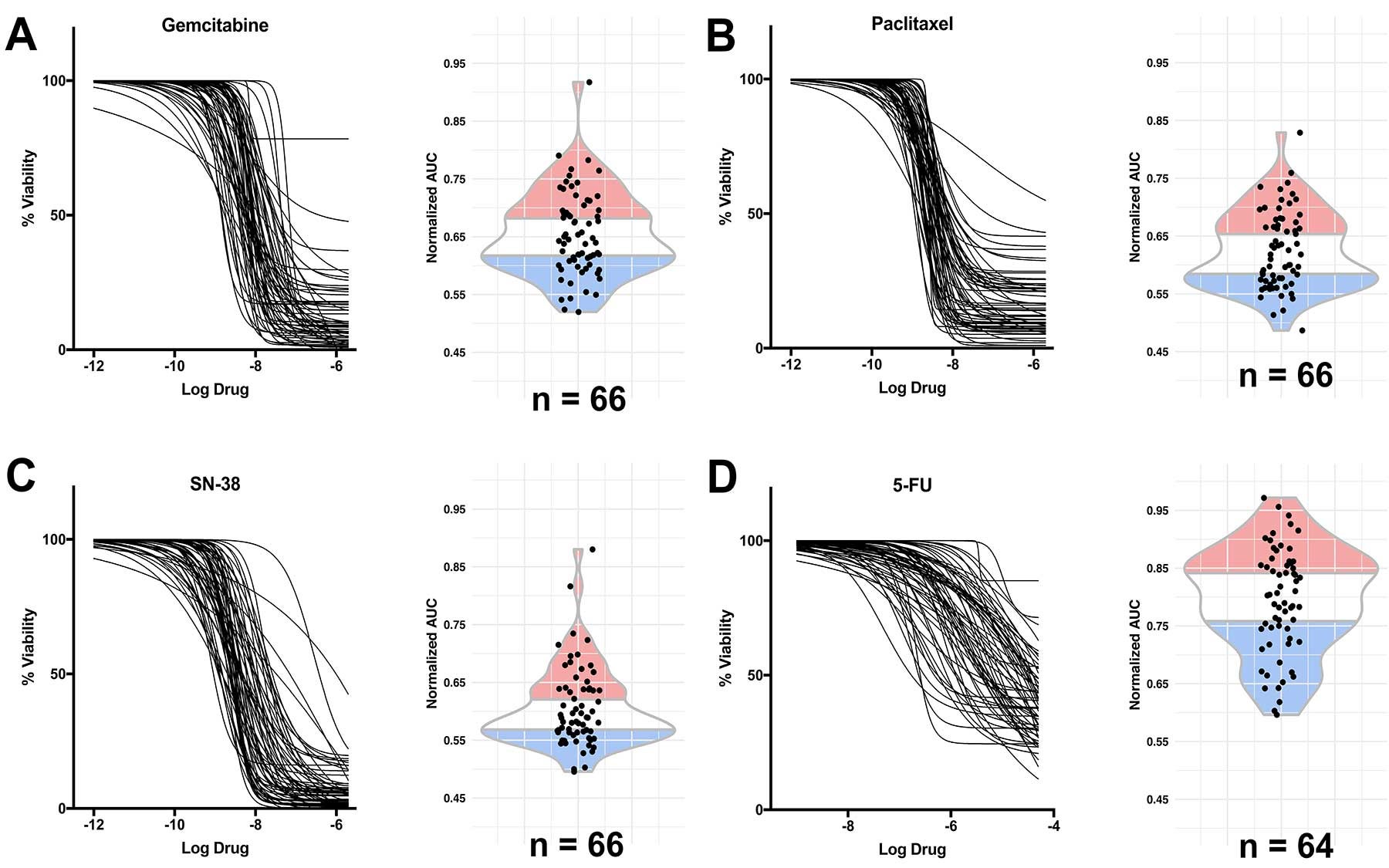
In this latest development, the Tuveson team generated a library of 66 organoids derived from pancreatic ductal adenocarcinoma (PDAC) tumor specimens at various stages of the disease. The researchers demonstrated that the organoids provide an effective precision-medicine “pharmacotyping,” or drug-testing, pipeline. To do this, “we culture the organoid from the patient’s cancer and then test all possible standard-of-care drugs as well as experimental drugs,” Tiriac explains.
The team assessed RNA levels in individual organoids—a way to measure gene activity—to predict the sensitivity of an organoid to the five chemotherapies currently administered to pancreatic cancer patients. They found that three so-called “signatures” of gene activity in the organoids correctly identified patients who had responded well to these drugs. “The signatures should enable physicians to choose the best initial chemotherapy treatment for pancreatic cancer,” Tuveson says.
Tuveson and his team plan to further refine the gene signatures through additional experiments, and to test in clinical trials the ability of signatures found in organoids to predict the responses of pancreatic cancer patients.
Written by: Chris Palmer, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
Lustgarten Foundation; National Institutes of Health; National Cancer Institute; V Foundation; Stand Up to Cancer; Precision Medicine Research Associates; SWOG ITSC; Pancreatic Cancer Action Network-AACR; State of New York; Concetta Greenberg in memory of Marvin S. Greenberg, M.D.; AACR Pancreatic Cancer Action Network; ASGE Endoscopic Research Award; Simons Foundation Award; Ontario Institute for Cancer Research; University Health Network BioBank; Ontario Institute for Cancer Research; Ontario Institute for Cancer Research, Canadian Cancer Society Research Institute; Canadian Friends of the Hebrew University; Pancreatic Cancer Canada Foundation; Lebovich Chair in Hepatobiliary/Pancreatic Surgical Oncology.
Citation
Tiriac H et al, “Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer” appears online in Cancer Discovery May 31, 2018.
Principal Investigator

David Tuveson
Professor
Roy J. Zuckerberg Professor of Cancer Research
Cancer Center Director
M.D., Ph.D., Johns Hopkins University, 1994

2 thoughts on “Organoid profiling personalizes treatments for pancreatic cancer”
Comments are closed.