Cold Spring Harbor Laboratory (CSHL) and The Feinstein Institutes for Medical Research are recruiting patients in an entirely virtual, at-home, randomized, double-blind, placebo-controlled clinical trial. The study evaluates the safety and efficacy of the drug famotidine, or PEPCID, for the outpatient treatment of COVID-19 in adults. Tobias Janowitz, M.D., Ph.D., a medical oncologist, assistant professor at CSHL, and adjunct professor at the Feinstein Institutes, is the principal investigator leading the trial. The study requires 84 patients to achieve statistical significance.
This is the first time strategic affiliates CSHL and Northwell Health have initiated an entirely virtual clinical trial which demonstrates the power of collaboration between scientists and clinicians. Northwell, New York’s largest health system, has treated more than 100,000 COVID-19 patients. The famotidine trial is recruiting patients across the greater New York area who have tested positive for COVID-19 but are experiencing only mild to moderate symptoms that do not require hospitalization.
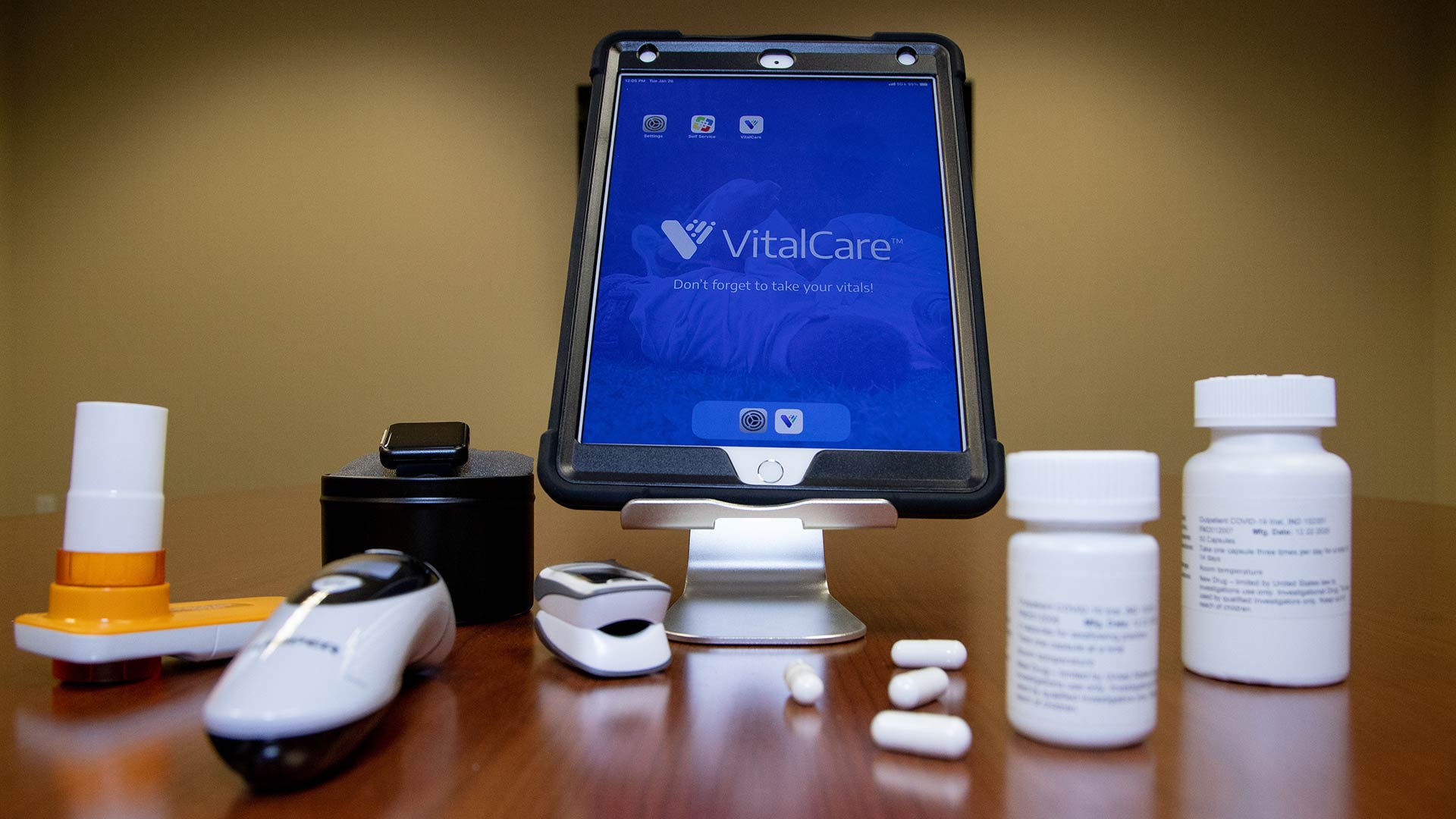
Janowitz and colleagues designed this study to keep trial participants safe and out of the hospital throughout their treatment. Patients will use a cellular-activated Apple iPad from their home to report on the severity of their symptoms every day. In addition, they will use a Bluetooth-enabled scale, thermometer, fitness tracker, spirometer (to study airflow in and out of the lungs), and pulse oximeter (to measure blood oxygen levels) to monitor their vital signs. Patients will swallow either a high-dose famotidine pill or one that looks just like it for a maximum of 14 days. Northwell’s Home Lab program will visit the patients to draw blood and administer COVID-19 diagnostic nasal swabs tests.
“From the comfort of these patients’ own homes, we are taking the traditional clinical trial completely digital to study the efficacy and safety of a potential COVID-19 therapy,” said Janowitz. “From assessment to enrollment and daily data collection, we hope this study’s model will be an example for future clinical trials and will provide high-quality data as we assess candidate treatments, like famotidine, to curb this disease.” Janowitz modeled this trial on methods used in treating cancer patients, where patients self-report symptoms.
Famotidine is a common, safe over-the-counter drug used to treat heartburn and assist with healing gastrointestinal ulcers. It is a histamine 2 blocker sold under the brand PEPCID. Janowitz and his colleagues reported on the results of their first virtual quantitative symptom tracking study of famotidine, a proof of concept, in June 2020.
At the start of the pandemic, the Feinstein Institutes established a COVID-19 Clinical Trial Unit (CCTU), a 200-member rapid-response clinical trial group of scientists, physicians, administrators, and staff to review and launch clinical trials. Since March, the CCTU has enrolled patients in more than seven clinical trials and programs—including famotidine, remdesivir, sarilumab, and convalescent plasma. Overall, more than 1,200 patients were enrolled into COVID-19 research trials across the health system, resulting in more than 200 manuscripts published, providing guidance to clinicians worldwide.
Written by: Dagnia Zeidlickis, Vice President, Communications | zeidlick@cshl.edu | 516-367-8455
Funding
The Fast Grant and Pershing Square Foundation.
Citation
https://clinicaltrials.gov/ct2/show/NCT04724720?term=famotidine&draw=1&rank=5
About

Tobias Janowitz
Associate Professor
Cancer Center Program Co-Leader
M.D., Ph.D., University of Cambridge, UK, 2007