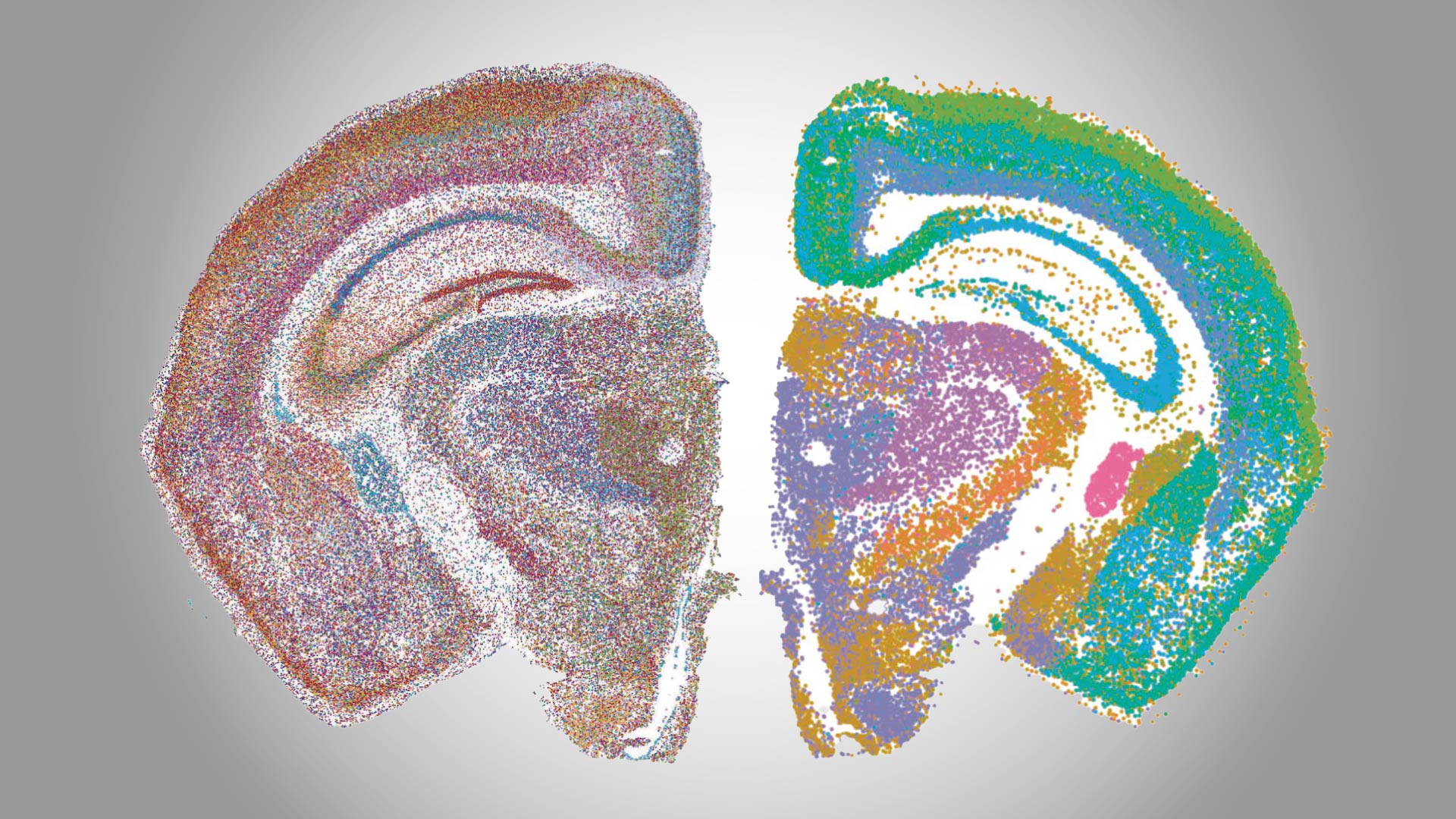
The brain is the most complex and powerful part of the human body. When things are working as they should, we don’t notice at all. However, any number of conditions can disrupt healthy brain function.
Cold Spring Harbor Laboratory (CSHL) neuroscientists focus on understanding how neural connections in the brain translate into behavior. Their research has yielded new insight into the biology underlying complex cognitive processes and what happens when things go wrong.
In celebration of World Brain Day, here is a sampling of CSHL’s latest discoveries in brain development and neurological conditions, from autism to Alzheimer’s disease.
Finding the sweet spot in brain development
As our brains assemble, trillions of neural connections have to be built or torn down at the right time and place. Otherwise, the seeds of disorders like autism can take root. Short-lived neural connections in the mouse brain help prime sensory circuits, forever affecting the mouse’s sense of touch. CSHL Assistant Professor Gabrielle Pouchelon and her team have discovered that a receptor protein named mGluR1 helps regulate the timing of these temporary connections.
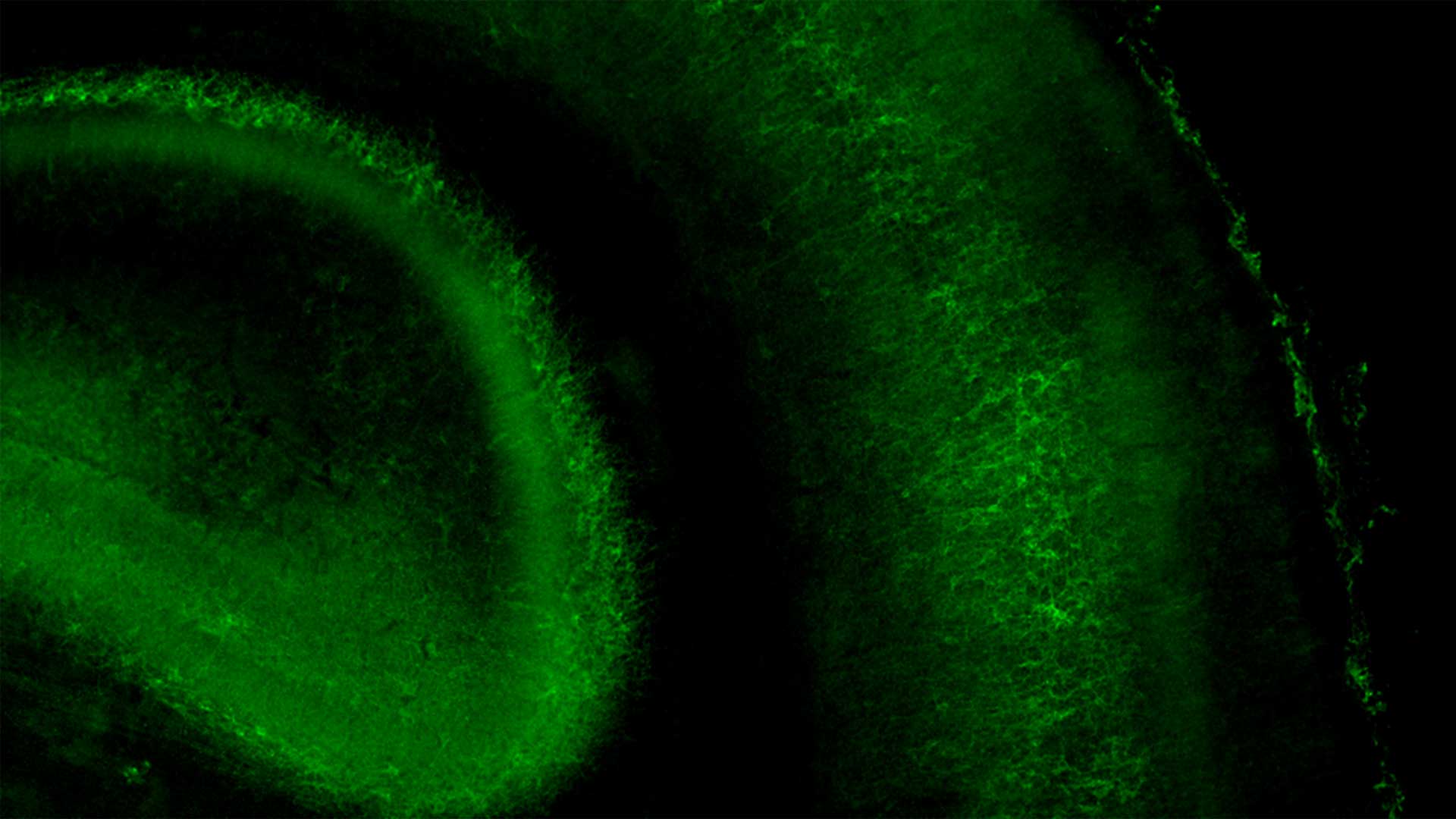
The team hopes their work may one day serve as a guide for new therapeutic strategies to treat any number of neurological and developmental conditions.
This protein does “The Twist”
Proteins are constantly performing a kind of dance. They move and contort their bodies to fulfill specific functions inside our bodies. The NMDA receptor (NMDAR) protein executes an especially hard dance routine in our brains. One misstep can lead to a range of neurological disorders. The problem is that scientists couldn’t figure out the last move in NMDAR’s routine—until now.

CSHL Professor Hiro Furukawa and his team have deciphered the critical step in which NMDAR rotates into an open formation. This may one day lead to drug compounds that can teach the correct moves to NMDARs that have lost a step. Better drugs that target NMDARs might have applications for neurological disorders like Alzheimer’s and depression.
Products of an unseen environment
What causes autism? Studies suggest that viral infection in pregnant women may play a role. However, many questions remain. How does this work? When is the risk greatest? Perhaps most tantalizing, why do some fetuses appear more vulnerable than others? Today, answers are beginning to emerge.
CSHL’s Irene Sanchez Martin, a postdoc in Assistant Professor Lucas Cheadle’s lab, points to recent experiments in which a mouse embryo may begin to show early signs of developmental deficits soon after its pregnant mother is exposed to a virus. This is the first time scientists have been able to look at the effects of prenatal inflammation on an embryo in an autism model. Such research may one day enable physicians to identify early warning signs before a child is even born.
The brain’s landscapers
The visual cortex processes everything we see. Incoming visual information is relayed to this outer layer of the brain via synapses. When the brain’s neural circuits are first wired up, more synapses are created than needed. As the brain accumulates new experiences and information, cells called OPCs shape neural circuitry by pruning unnecessary synapses. These mysterious cells are also involved in the development of glioma, a deadly brain cancer, and may play a role in Alzheimer’s.

CSHL Associate Professor Lucas Cheadle has developed new ways to zoom in and see OPCs in action. “From there, we can figure out which synapses are fully engulfed by an OPC, which are in the process of being pruned, and which have maybe just been checked on by an OPC but not processed,” he explains.
It’ll take more research to illustrate these connections in detail. In the meantime, Cheadle is eager to share his lab’s new tools with researchers around the world.
Putting out a brain on fire
Anti-NMDAR encephalitis is a rare autoimmune disorder that may be misdiagnosed as schizophrenia. It can lead to hallucinations, blackouts, and psychosis, says CSHL Professor Hiro Furukawa. It mostly affects women ages 25 to 35—the same age at which schizophrenia often presents itself. But what’s happening in anti-NMDAR encephalitis is something else.
Furukawa has shown how three patients’ antibodies bind to crucial brain receptors in different cases of this immune disease. The discovery suggests that personalized medicine is crucial for more accurate treatments and diagnoses.
Mixed signals: How the brain interprets social cues
Exactly how sensory signals mix and influence each other in the brain isn’t well understood. To shed light on the subject, CSHL Professor Stephen Shea and graduate student Alexandra Nowlan traced how smell and hearing interact in mouse brains during a maternal behavior called pup retrieval.

Shea and his team found that smell and sound signals merge in the mouse brain’s hearing center, influencing social and maternal behaviors. The discovery may lead to a better understanding of how neurological conditions such as autism affect a person’s ability to interpret social cues.
A hearing aid for … your nose?
CSHL Professor Florin Albeanu and CSHL postdoc Diego Hernandez Trejo have discovered a feedback loop in the brain’s odor center that seems to put smells and sounds into context. Their findings point to never-before-seen fast-updating signals between the brain’s olfactory cortex and olfactory bulb. The feedback loop may enable an animal’s brain to immediately adapt to changes and help the animal fine-tune its motor responses accordingly.
The discovery tracks with the Shea lab’s aforementioned findings, which shows how sensory cues become integrated with each other in the brain. It also raises some exciting questions about the world we share and the perceptions that shape our understanding of it.
Ketamine: From club drug to antidepressant?
“It’s been suggested for over a decade that the drug ketamine blocks a specific kind of NMDA receptor, called GluN1-2B-2D,” says CSHL Professor Hiro Furukawa.
But there was one big problem with this suggestion. Scientists weren’t quite sure that GluN1-2B-2D existed. A study from the Furukawa lab shines much-needed light on the situation. In a paper published in the journal Neuron, Furukawa and postdoc Hyunook Kang prove that GluN1-2B-2D does exist in the mammal brain. Their research may lead to safer treatments for depression, anxiety, and other mental health conditions.
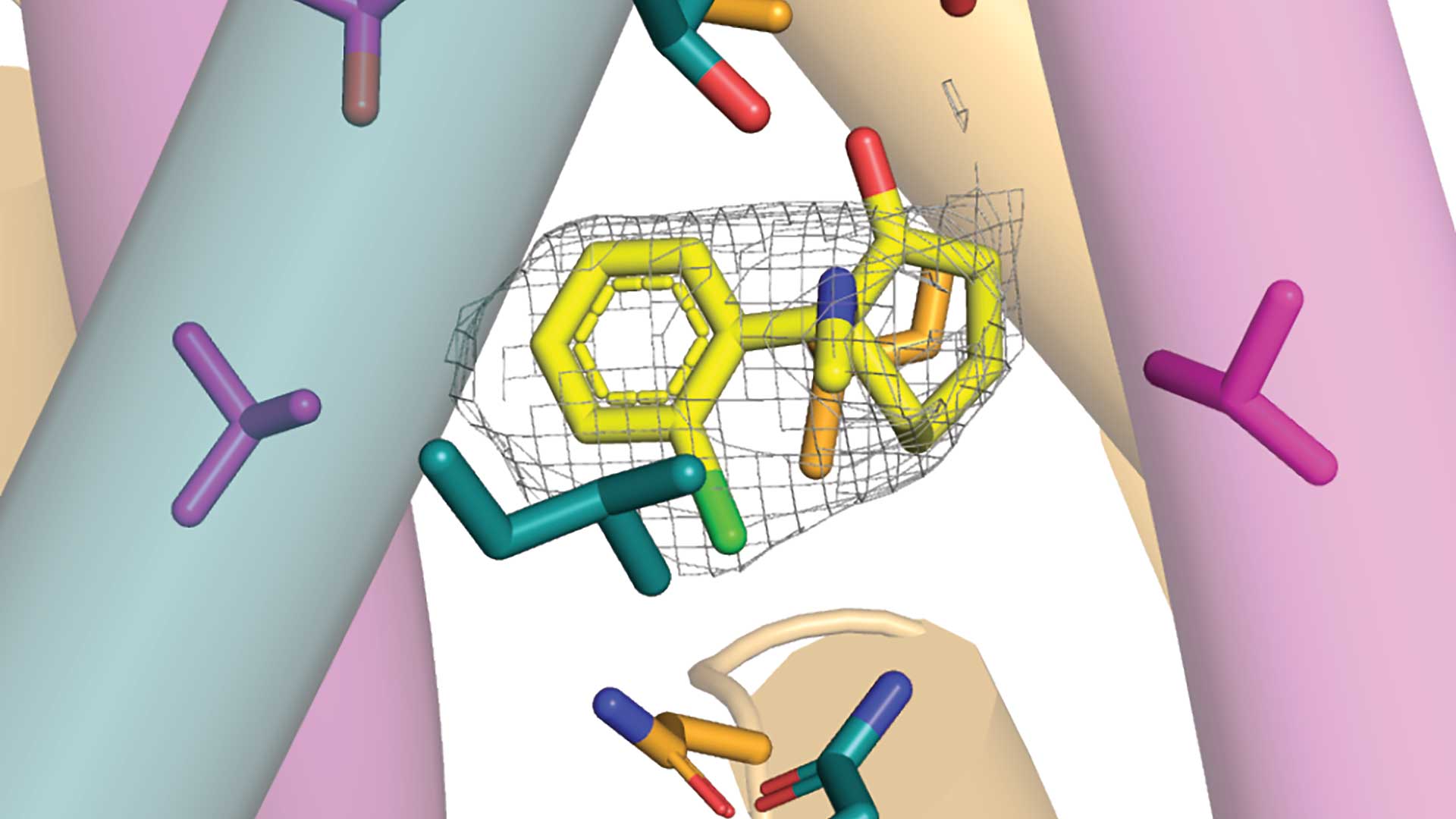
It’s thought that ketamine may ease symptoms of depression and anxiety by affecting GluN1-2B-2D movement. Side effects range from mild hallucinations to full-on psychosis. However, if scientists can determine how GluN1-2B-2D movements affect the brain, they may be able to synthesize new versions of the drug with fewer harmful side effects.
It’s not you—it’s cancer
CSHL Associate Professor Tobias Janowitz and colleagues have identified a circuit connecting the brain and immune system that may be responsible for the sense of apathy many late-stage cancer patients experience. Their research, published in Science, suggests apathy and lack of motivation are symptoms of a severe wasting condition called cancer cachexia.
The team, including CSHL alumni Adam Kepecs, discovered a full brain circuit that senses inflammation in the bloodstream and sends signals to the brain that reduce motivation. The discovery reveals that apathy isn’t just an emotional or psychological reaction to cachexia. It’s built into the biology of the disease.
The team’s results suggest that existing antibody treatments could be repurposed to improve cancer patients’ quality of life and enable them to better tolerate common cancer therapies.
A new body of knowledge
Cancer neuroscience is a field so new, there isn’t even a degree program for it yet. Still, the potential implications for cancer patients and research are staggering. So, for scientists interested in studying this emerging field, the question becomes where to begin. How do you pick up the tools of the trade when they haven’t been established yet?
You come to Cold Spring Harbor. Held at CSHL from March 3 to March 19, Methods in Cancer Neuroscience became the burgeoning field’s first-ever hands-on course. Over an intense two weeks, participants received training in a wide variety of techniques. Each attendee gained experience studying the influence of nerves on cancer from the organism level down to the cellular level. From there, students were able to take what they’d learned back home to teach others and help build up the field. Through these efforts, the new research area will keep growing in size, reach, and impact.
Likewise, CSHL continues to expand its multipronged neuroscience program. To find out more, check out our neuroscience archive. And keep an eye out for future additions to this ever-growing body of knowledge.
Written by: Communications Department | [email protected] | 516-367-8455