When researchers look for potential cancer therapy targets, they typically go after protein-coding genes that participate in disease progression. Disrupt them and negate whatever role they play in cancer’s development or spread. Researchers at Cold Spring Harbor Laboratory (CSHL) have also been working on another kind of target. They’re studying a long non-coding RNA called MALAT1. So far, it’s been linked to more than 20 different types of tumors.
In a new and unique study published in Molecular Therapy: Oncology, CSHL researchers track MALAT1 levels over the course of one woman’s experience with triple-negative breast cancer (TNBC). They find that MALAT1 levels were high at diagnosis and decreased while the patient received standard treatments. Yet, perhaps most importantly, the level increased at a distant metastatic site. The researchers see this as supporting a role for MALAT1 in TNBC’s spread.
The study allowed CSHL to interrogate this potential treatment target like never before. “Even though MALAT1 has been implicated in different cancers, including breast cancer, nobody has looked at how MALAT1 levels change over treatment and disease progression,” says Disha Aggarwal, the graduate student who spearheaded the study.
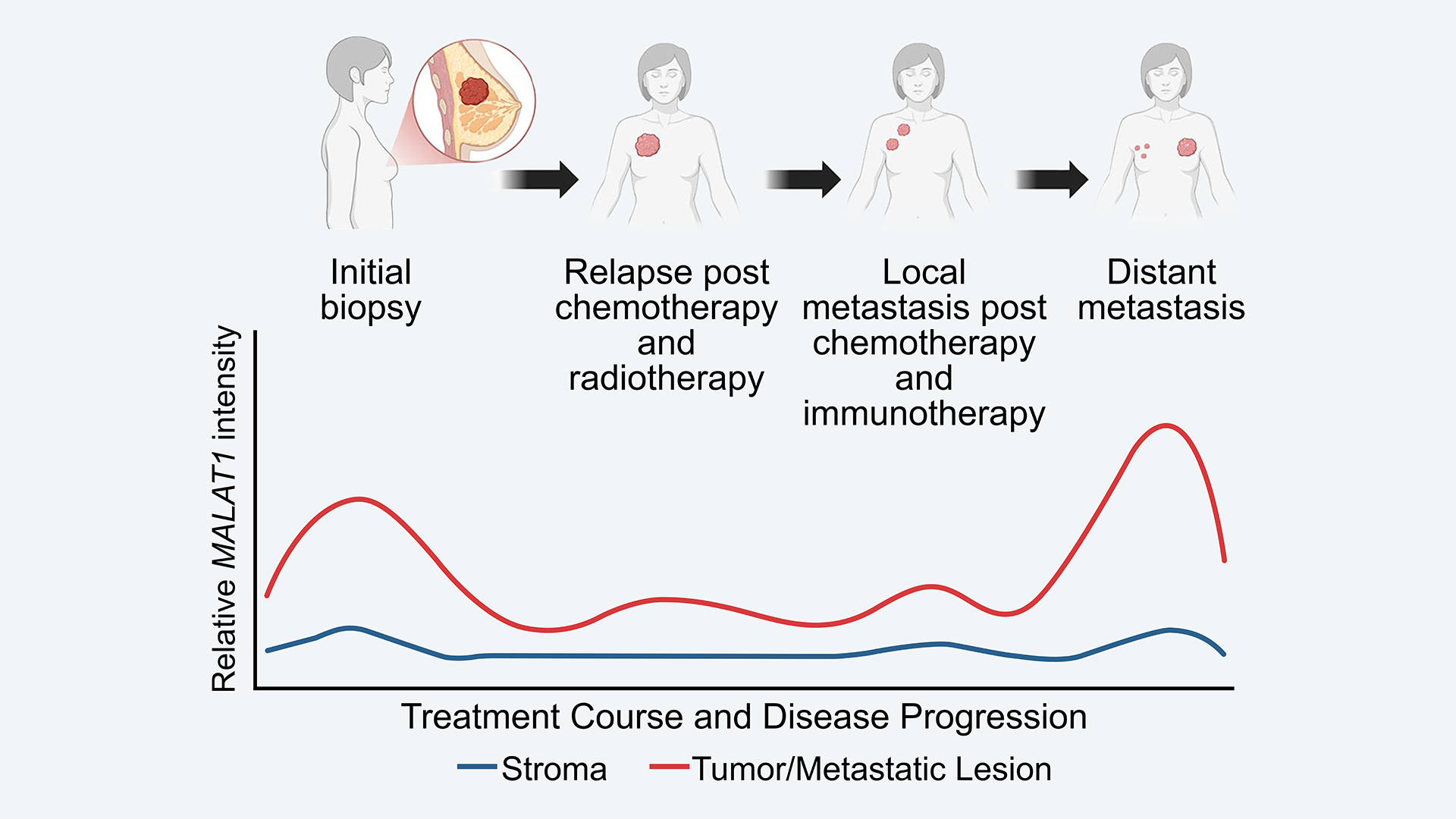
Researchers examined tissue samples from a 59-year-old woman diagnosed with stage 1 TNBC. The patient underwent different treatments over two and a half years, including surgery, chemotherapy, radiation, and immunotherapy. After a period of regression, the cancer became metastatic. Unfortunately, three and a half years after diagnosis, the individual succumbed to the disease. However, what she left behind will hopefully help others.
Studying tissue samples from throughout the course of treatment and disease progression presented a rare opportunity, says CSHL Professor David Spector. “Researchers typically get to see an initial sample and an end sample, but not progressive samples in the depth that we were able to look at here.” What they’ve seen may eventually inform future treatments.
Since 2015, the Spector lab has been collaborating with Ionis Pharmaceuticals to develop a drug targeting MALAT1. They’re now speaking with biotech companies in hopes of launching a clinical trial within the next few years. But that’s not all.
Spector’s lab is also exploring whether MALAT1 could enable doctors to predict the risk of someone’s cancer recurring or becoming metastatic. If so, it could not only guide therapeutic strategies for women who have been diagnosed with TNBC. It could factor in for those with more common and less severe forms of breast cancer.
Written by: Jen A. Miller | publicaffairs@cshl.edu | 516-367-8455
Funding
National Institutes of Health, National Cancer Institute, CSHL-Northwell Health Affiliation
Citation
Aggarwal, D., et al., “Longitudinal tracking of MALAT1 level over a breast cancer patient’s course of treatment and disease progression”, Molecular Therapy: Oncology, October 17, 2025. DOI: 10.1016/j.omton.2025.201070
Core Facilites
Principal Investigator

David L. Spector
Professor
Robert B. Gardner Jr., Professor
Cancer Center Member
Ph.D., Rutgers University, 1980
