A molecule called NADP regulates a cascade enabling yeast cells to adjust metabolic state
Cold Spring Harbor, NY — Metabolism is a central feature of life—a myriad of biochemical processes that, together, enable organisms to nourish and sustain themselves. Scientists at Cold Spring Harbor Laboratory (CSHL) are at the forefront of efforts to demonstrate how the regulation of genes governs fundamental life processes, including metabolism.
Such research, performed on simple model organisms like yeast cells, has implications for efforts to understand natural processes such as aging and disease states including cancer.
This week a team at CSHL led by Professor Leemor Joshua-Tor, Ph.D., announced a new and unexpected wrinkle in a story they previously thought they understood about how yeast cells, through the action of genes, adjust their metabolism in response to changes in their sources of food. The team’s findings were published February 22 in the journal Science.
Adapting to New Energy Sources
“S. cerevisiae, or common baker’s yeast, can use any number of different types of sugar molecules for energy production,” noted Dr. Joshua-Tor, a structural biologist. “Importantly, the yeast cell can rapidly respond to changes in its nutritional environment by altering the expression of specific genes that allow it to make use of those different energy sources.”
This much, notes Dr. Joshua-Tor and colleagues, has been understood for years. “The players involved in this process have been known for some time. But we did not understand precisely how the components of this particular biochemical pathway worked together,” said Stephen Johnston, a professor at the Biodesign Institute at Arizona State University and a co-author of the study.
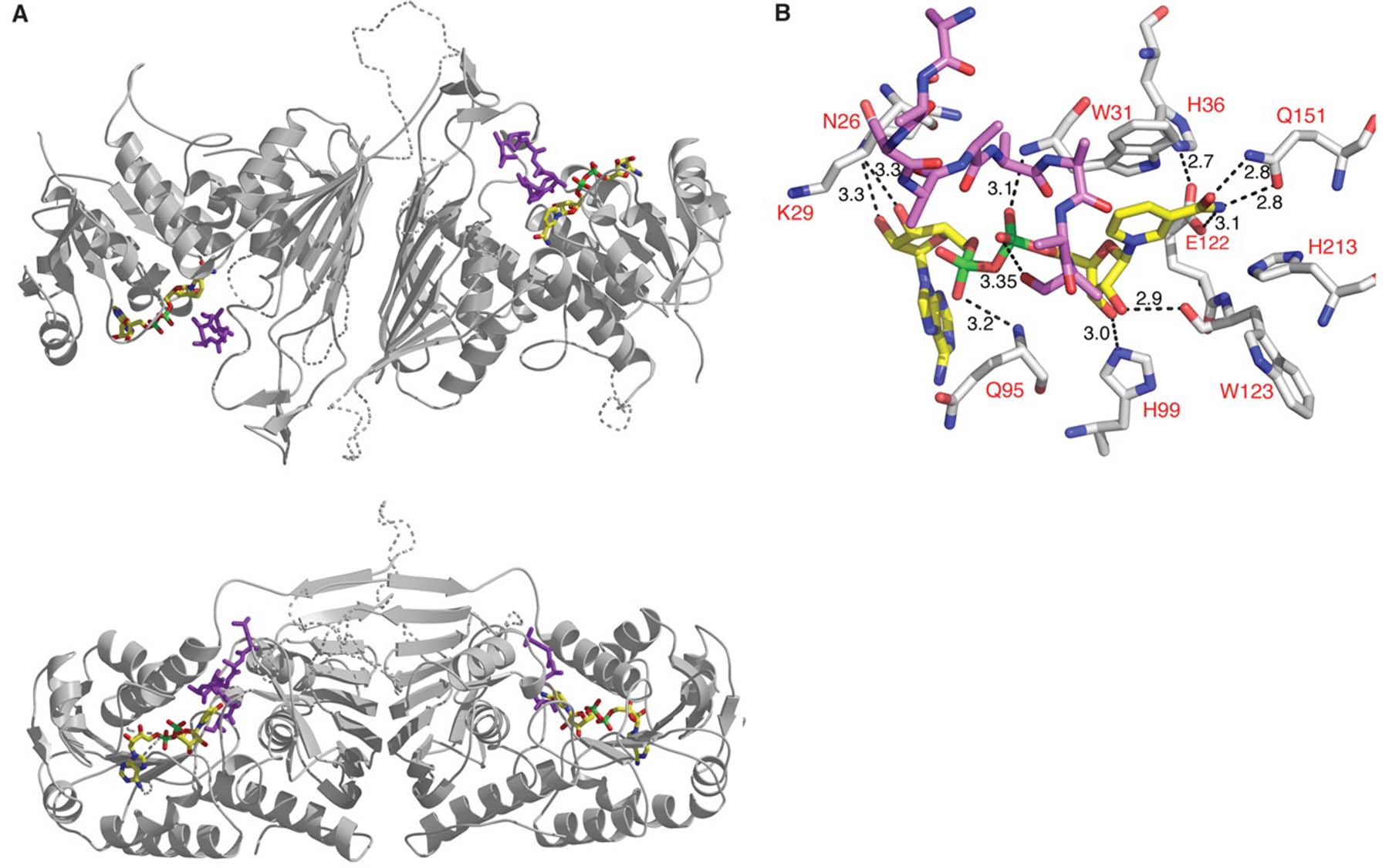
It was Dr. Joshua-Tor’s team at CSHL that took the step of investigating the architecture of the proteins involved in the pathway, at the level of individual atoms. Using a technique called x-ray crystallography, they discovered a “player” in the molecular cast of characters whose involvement previously had been overlooked.
The unexpected molecule is called NADP. The team discovered that when a yeast cell changes from using glucose, a simple sugar, as a nutritional source to using galactose, a more complex sugar often found in dairy products and vegetables such as sugar beets, NADP is called into action. It “docks” to a protein called Gal80p, which acts along with a gene regulating-protein called Gal4p, to adapt the metabolism of the yeast cell so that it can make use of galactose.
“Importantly, changes in cellular levels of NAD, a close relative of NADP, had previously been linked to a gene circuit that controls aging and longevity in a large number of different organisms, including yeast but also including animals,” said Professor Rolf Sternglanz of Stony Brook University in New York, a co-author of the study.
Why The Regulatory Cascade Is Important
“It is becoming increasingly clear that the metabolic state of a cell is linked to the expression of its genes in a way that impacts biological processes of many kinds, ranging from cancer to aging,” said Dr. Joshua-Tor. The biochemical cascade identified by the team is part of a complex chain of events whose object is regulation of the output of specific genes.
Not only does the team’s work help explain how links in that gene-regulatory chain are constructed. “Gene-regulatory proteins impact every property of a cell and have long been recognized as possible targets for drugs,” said Dr. Joshua-Tor. “However, these types of proteins have proven resistant to the chemistry of modern drug design. A detailed understanding of how gene regulatory proteins are controlled may offer new and unanticipated opportunities to design drugs that would impact this class of proteins.”
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455
Citation
“NADP Regulates the Yeast GAL Induction System” appears in Science on February 22. The compete citation is as follows: P. Rajesh Kumar, Yao Yu, Rolf Sternglanz, Stephen Albert Johnston, Leemor Joshua-Tor. The paper is available online here.
Principal Investigator

Leemor Joshua-Tor
Professor, Director of Research & HHMI Investigator
W.M. Keck Professor of Structural Biology
Cancer Center Member
Ph.D., The Weizmann Institute of Science, 1991