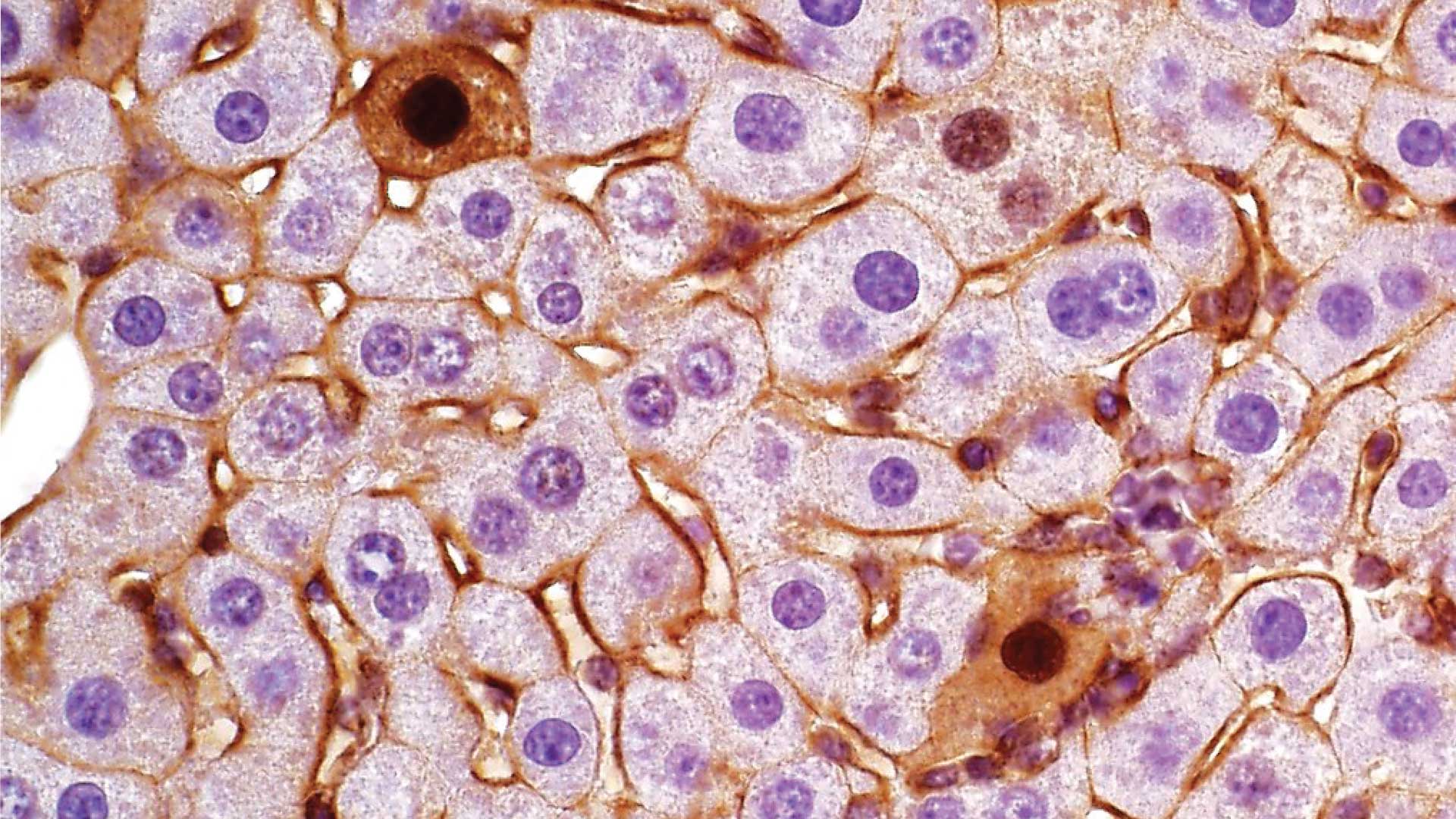
Mutations in our genes can lead to severe problems, like colon or liver cancer. But cancer is very complex. Mutations in the same genes can lead to different subtypes of tumors in different people. Currently, scientists don’t have a good way to produce such tumor subtypes for study in the lab.
Now, Cold Spring Harbor Laboratory Assistant Professor Semir Beyaz has created a new method to model certain liver cancer tumor subtypes using the gene-editing tool CRISPR-Cas9.
Genes contain the information our bodies need to create proteins. Highly similar proteins produced from the same gene are called isoforms. Different isoforms generate different tumors. This process is known as exon skipping, where multiple parts of a gene are stitched together to make a different version of a protein.
“Everyone thinks that cancer is just one type,” Beyaz explains. “But with different isoforms, you can end up with cancer subtypes that have different characteristics.”
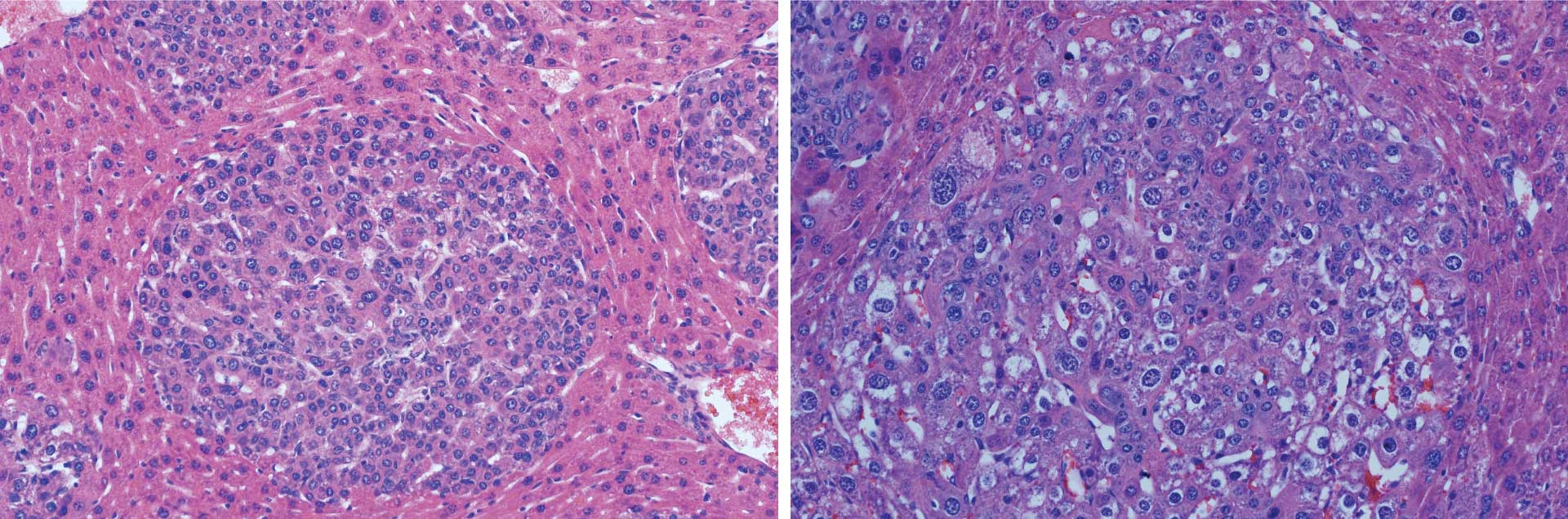
Beyaz and his colleagues produced two distinct tumor subtypes by targeting a single section of the mouse gene, Ctnnb1, with CRISPR. The tool is mostly used to inhibit gene function. This is the first time CRISPR has been used to generate different cancer-causing gain-of-function mutations in mice. These mutations enhance protein activity to promote tumor growth. The team sequenced each tumor subtype to figure out which isoform was associated with the differences they observed.
“We were able to define those isoforms that associated with different cancer subtypes,” Beyaz says. “That was, for us, a surprising discovery.”
Next, to confirm that these isoforms actually caused the variances, they produced them in the mouse without using CRISPR. They found that they were indeed able to generate the two different tumor subtypes with their respective characteristics. Both of these liver tumor subtypes are also found in humans.
The mutations Beyaz targeted can lead to colon and liver cancers. Targeting exon skipping has emerged as a potential therapeutic approach for treating cancer and other diseases. Beyaz’s new study method allows researchers to investigate this phenomenon in living mice cells using CRISPR. The platform could someday help researchers develop new therapeutic interventions. “Ultimately,” Beyaz explains, “what we want to do is find the best models to study the biology of cancer so that we can find a cure.”
Written by: Margaret Osborne, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
National Cancer Institute, National Institutes of Health, Mark Foundation for Cancer Research, Oliver S. and Jennie R. Donaldson Charitable Trust, STARR Cancer Consortium, National Center for Advancing Translational Sciences, and Swedish Research Council
Citation
Beyaz, S., et al., “CRISPR-induced exon skipping of β-catenin reveals tumorigenic mutants driving distinct subtypes of liver cancer”, The Journal of Pathology, January 15, 2023. DOI: 10.1002/path.6054
Principal Investigator

Semir Beyaz
Assistant Professor
Cancer Center Member
Ph.D., Harvard University, 2017