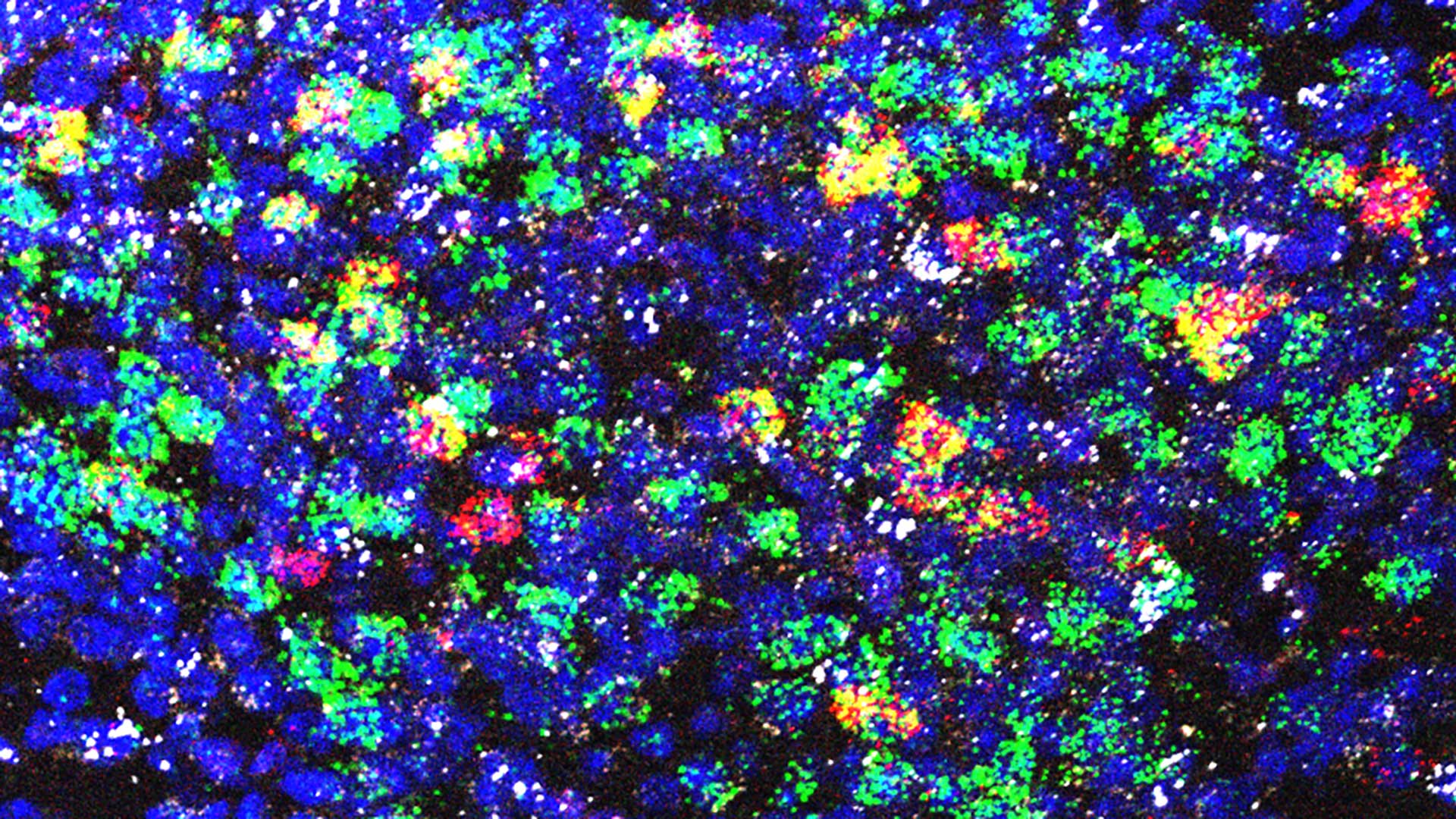
Cancer is insidious. Throughout tumor progression, the disease hijacks otherwise healthy biological processes—like the body’s immune response—to grow and spread. When tumors elevate levels of an immune system molecule called Interleukin-6 (IL-6), it can cause severe brain dysfunction. In about 50%-80% of cancer patients, this leads to a lethal wasting disease called cachexia. “It’s a very severe syndrome,” says Cold Spring Harbor Laboratory (CSHL) Professor Bo Li. He explains:
“Most people with cancer die of cachexia instead of cancer. And once the patient enters this stage, there’s no way to go back because essentially there’s no treatment.”
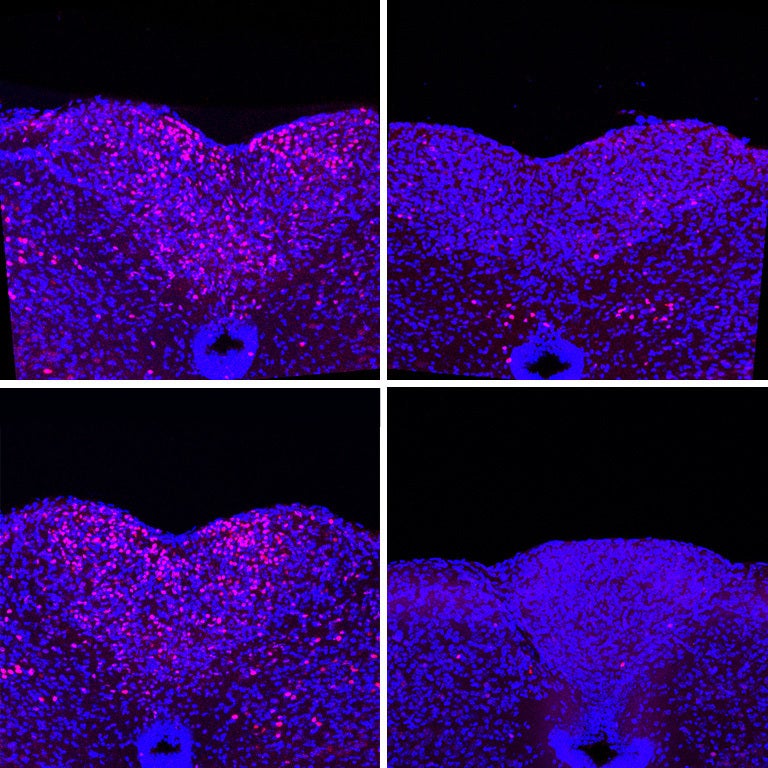
Now, Li and a team of collaborators from four CSHL labs have found that blocking IL-6 from binding to neurons in a part of the brain called the area postrema (AP) prevents cachexia in mice. As a result, the mice live longer with healthier brain function. Future drugs targeting these neurons could help make cancer cachexia a treatable disease.
In healthy patients, IL-6 plays a crucial role in natural immune response. The molecules circulate throughout the body. When they encounter a possible threat, they alert the brain to coordinate a response. Cancer disrupts this process. Too much IL-6 gets produced, and it begins binding to AP neurons in the brain. “That leads to several consequences,” Li says. “One is animals and humans alike will stop eating. Another is to engage this response that leads to the wasting syndrome.”
The team took a two-pronged approach to keeping elevated IL-6 out of the brain in mice. Their first strategy neutralized IL-6 with custom antibodies. The second used CRISPR to reduce the levels of IL-6 receptors in AP neurons. Remarkably, both tactics produced the same results—the mice started eating again, stopped losing weight, and lived longer.
For Li, the implications were mind-blowing:
“The brain is so powerful in regulating the peripheral system. Simply changing a small number of neurons in the brain has a profound effect on whole-body physiology. I knew there was an interaction between tumors and brain function, but not to this extent.”
Li says his team is now determined to figure out how to translate this discovery to human patients. Their recent collaborations with CSHL Professor Adrian Krainer and former CSHL Professor Z. Josh Huang may bring them one step closer. “If we can use what we’ve learned to prevent or treat cachexia, we can dramatically increase patients’ quality of life,” Li says. “This could one day have a big impact on many people.”
Written by: Nick Wurm, Communications Specialist | wurm@cshl.edu | 516-367-5940
Funding
The Lustgarten Foundation, Thompson Family Foundation, Pershing Square Foundation, CSHL-Northwell Health Affiliation, Northwell Health, Cold Spring Harbor Laboratory Association, National Institutes of Health, Simons Foundation, National Cancer Institute, Cancer Grand Challenges, Mark Foundation for Cancer Research, Osprey Foundation, Fortune Footwear, Key Research and Development Program of Zhejiang Province
Citation
Sun, Q., et al., “Area postrema neurons mediate interleukin-6 function in cancer cachexia”, Nature Communications, June 1, 2024. DOI: 10.1038/s41467-024-48971-1
Core Facilites
Principal Investigator

Bo Li
Professor
Robert Lourie Professor of Neuroscience
Ph.D., The University of British Columbia, 2003