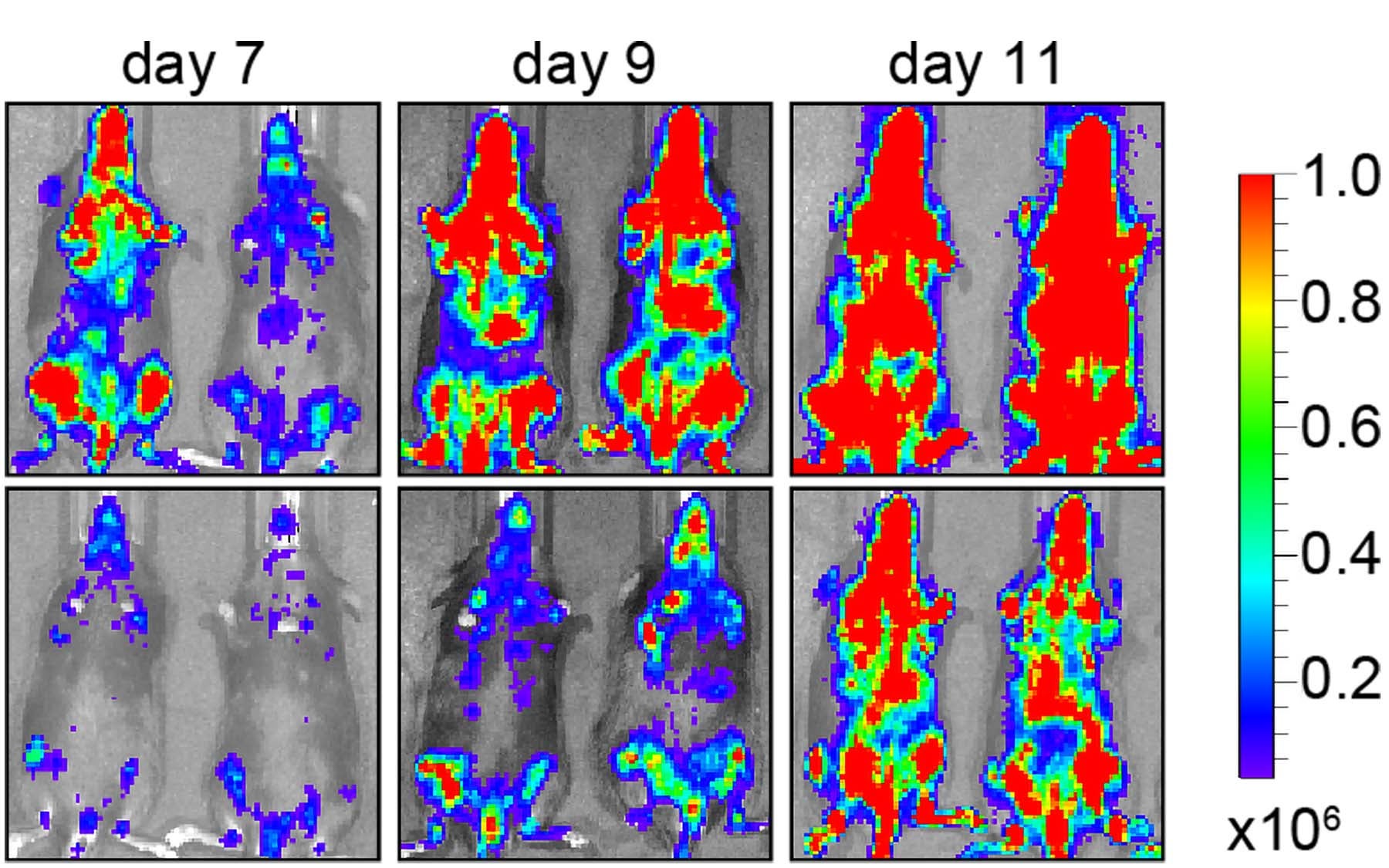
Cold Spring Harbor Laboratory scientists, with chemists and cancer biologists from Dana-Farber Cancer Institute (DFCI), have developed a new therapy that extended the survival of mice with acute myeloid leukemia.
The scientists are the first to demonstrate the anti-cancer effect of blocking the Salt-Inducible Kinase 3 (SIK3) pathway in leukemia using YKL-05-099, a drug developed within the lab of Nathanael Gray at DFCI. SIK3 is a kinase that controls cell division and survival of leukemia cells. Blocking SIK3 prevents leukemia cells from growing.
“Our experiments validate that pharmacological blockade of SIK3 is well-tolerated and extends the survival of leukemic mice,” said CSHL Professor Christopher Vakoc, who co-led the study with Kimberley Stegmaier at DFCI. Yusuke Tarumoto, a former postdoctoral researcher in Vakoc’s lab, and Shan Lu of Stegmaier’s lab are the co-first authors of the paper. Dr. Tarumoto is now a professor at Kyoto University. The team’s findings are published in the journal Blood.
Vakoc refers to this type of leukemia treatment as epigenetic therapy, which can change gene activity within the cancer cell. “Because epigenetics is a cellular system that is malleable and dynamic, it’s something that we can modulate with drugs,” he said. “Developing epigenetic cancer therapies is the core mission of my lab, with SIK3 inhibition in leukemia being our most recently developed strategy.”
In 2018, the Vakoc lab used CRISPR genetic screening to identify the SIK3 kinase as a non-obvious leukemia drug target.
“It’s an under-studied signaling molecule in the pathogenesis of cancer because it’s not mutated in cancer,” Vakoc said.
The subtype of leukemia the researchers focused on, MLL, is an aggressive form of cancer that occurs in infants and can be caused by an abnormal rearrangement of chromosomes, which is known as an MLL translocation. “We discovered SIK3 has a very important role in the MLL translocation positive subtype of leukemia,” Vakoc said.
The most important finding in this study is in revealing a drug development strategy for treating MLL leukemia.
The new compound created by the team to target SIK3 reprograms a transcription factor, which is a protein that can help turn specific genes on or off by binding to DNA. Vakoc’s lab has been at the forefront of trying to reprogram transcription factors in cancer therapy.
“It’s widely considered that this is impossible to do,” he said. “Our lab wants to challenge that idea.”
The study also helped researchers gauge the side effects of the drug. After using YKL-05-099 to suppress SIK3 in mice for a month, the researchers observed the drug to be well-tolerated, not causing any weight loss or significant changes to the animal’s normal blood production.
“We took a basic science idea that we published in a paper last year and now we’ve shown that it may have some usefulness in the clinic,” said Vakoc. “This new study advances our fundamental science towards clinical application, and it’s a very important milestone in that process.”
Written by: Charlotte Hu, Content Developer/Communicator | publicaffairs@cshl.edu | 516-367-8455
Funding
This research was funded by the CSHL NCI Cancer Center, the Forbeck Foundation, Pershing Square Sohn Cancer Research Alliance, National Institutes of Health, the Leukemia & Lymphoma Society, and the Lauri Strauss Leukemia Foundation.
Citation
Tarumoto et. al, “Salt-Inducible Kinase inhibition suppresses acute myeloid leukemia progression in vivo” appeared in Blood on November 1, 2019. https://doi.org/10.1182/blood.2019001576
Principal Investigator

Chris Vakoc
Professor
Alan and Edith Seligson Professor of Cancer Research
Cancer Center Deputy Director of Research
M.D., Ph.D., University of Pennsylvania, 2007