Protein named for comic book hero is no joke: it guides gene-silencing machinery to sites of havoc-causing transposons
Cold Spring Harbor, NY — Organisms from bacteria to humans must defend themselves against parasitic genetic elements called transposons, and the stakes are high. These pieces of DNA, which disrupt genes by jumping around in the genome, can cause so much destruction that cells have dedicated surveillance mechanisms to keep them in check.
To protect future generations against genomic havoc, defects in these innate defense systems usually result in sterility. In animals, the main defense against troublemaking transposons is the Piwi-interacting RNA pathway.
In new research, scientists led by Cold Spring Harbor Laboratory (CSHL) Professor Gregory Hannon, who is also a Professor and Senior Group Leader at the CRUK Cambridge Institute at the University of Cambridge, have identified a protein the Piwi system uses to guide a cell’s gene-silencing machinery to the right spots in the genome, allowing it to keep transposons inactive without interfering with the organism’s own genes.
The Piwi-interacting RNA pathway, which is active in germline cells—those that give rise to sperm and eggs—is a double-tiered defense system. The most direct line of defense finds and chews up RNA copies of transposons, while a second mechanism finds transposons within the genome and tags them with chemical signals that instruct the cell to keep them inactive. “These two modes of control provide the tightest possible clamp on the system,” Hannon says.
For the current study, Hannon and his colleagues focused on the system that chemically tags transposon DNA. This happens just as the elements are preparing to move, when transposon DNA is copied from its place in the genome into RNA, a process known as transcription.
The challenge for the Piwi system, Hannon explains, is to locate these sequences—which look a lot like an organism’s own genes—and tag them so they can be recognized by the same machinery a cell uses to switch its own genes off as needed. Small RNA molecules called Piwi-interacting RNAs are thought to be responsible for finding transposons as RNA copies emerge, but the next step was unexplained. “We wanted to find the link between this very transposon-specific pathway to the more general repressive machinery of the cell,” Hannon explains.
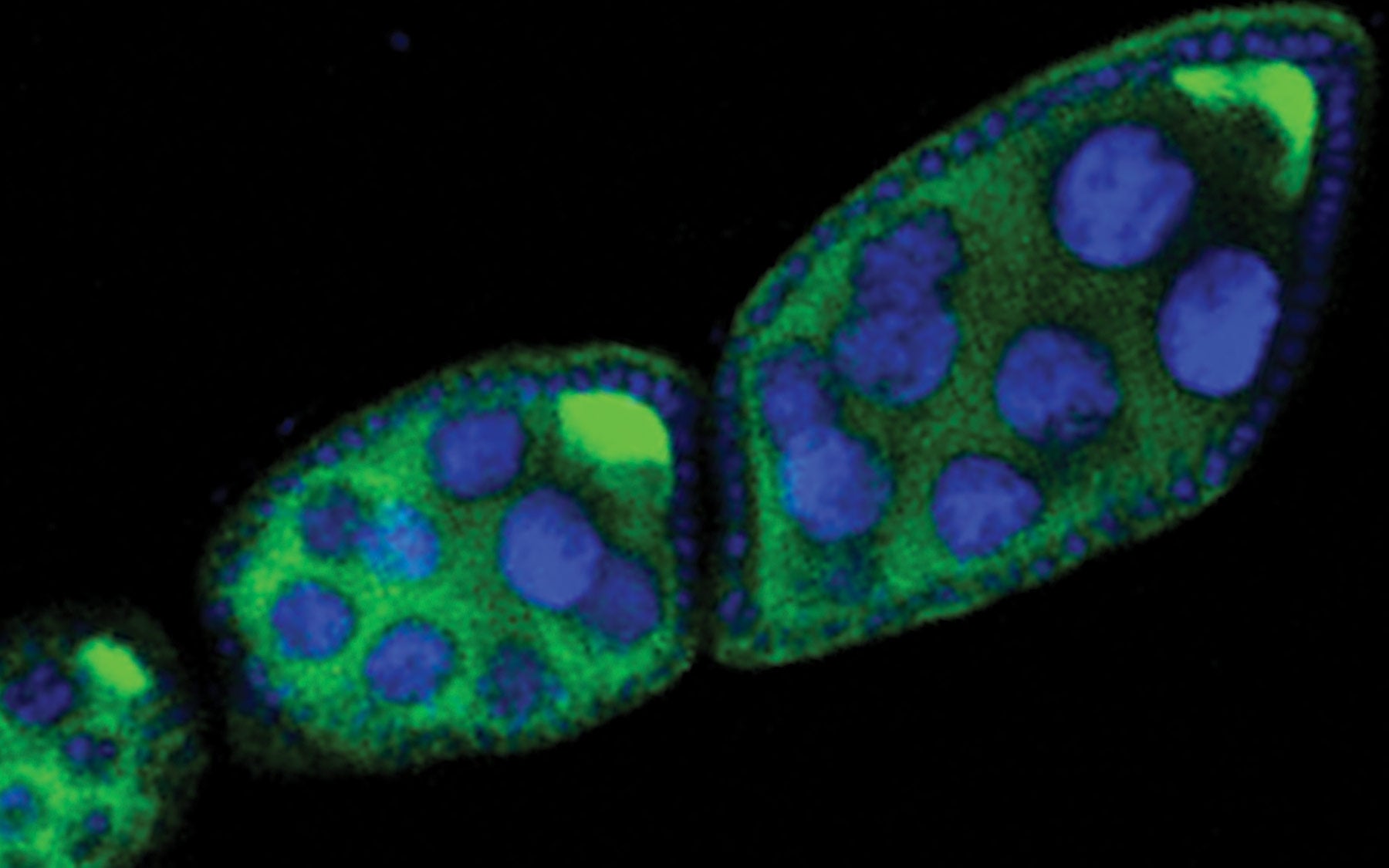
The scientists had a list of molecules they thought might play this role, and Yang Yu, a postdoctoral researcher in Hannon’s lab at CSHL, set out to test them. The Piwi system is so fast that scientists have found it nearly impossible to follow its components as they silence their natural targets. So Yu constructed an artificial Piwi target, then stuck different components of the Piwi system to it and monitored what happened next.
In most situations, cells treated the piece of DNA that encoded the target as one of their own genes. But when Panoramix was tethered to the artificial RNA target, it was able to halt transcription of the foreign piece of DNA. That suggested that as soon as transcription began, Panoramix recruited the cell’s gene silencing machinery to shut off local transcription and mark the intrusive DNA so that it would remain off, even in future generations.
“This is an incredibly specific phenomenon,” Hannon says, noting that Panoramix summons the cell’s gene-silencing machinery without affecting the activity of any of the fruit fly’s own genes.
Although no Panoramix protein has been found in mammals, Hannon says it is very likely that humans and other animals have a related protein that carries out the same role.
Written by: Jennifer Michalowski, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
This work was supported by the National Institutes of Health, the American Heart Association, HHMI and Cancer Research UK.
Citation
“Panoramix enforces piRNA-dependent cotranscriptional silencing” appears in Science on October 15, 2015. The authors are: Yang Yu, Jiaqi Gu, Ying Jin,Yicheng Luo, Jonathan B. Preall, Jinbiao Ma, Benjamin Czech, and Gregory J. Hannon. The paper can be obtained online at: http://www.sciencemag.org/journals