PLoS Biology publishes “Enhancement of SMN2 Exon 7 Inclusion by Antisense Oligonucleotides Targeting the Exon”
Cold Spring Harbor, NY — RNA splicing antisense technology studied at Cold Spring Harbor Laboratory (CSHL) effectively corrected an mRNA splicing defect found in spinal muscular atrophy (SMA) patients, and is now ready to be tested in mouse models. “SMA patients who suffer from motor-neuron degeneration may benefit from our ability to correct the mRNA splicing defect that makes their SMN2 genes only partially functional,” suggested CSHL Professor Adrian Krainer, Ph.D.
RNA splicing antisense technology allows researchers to influence the ultimate structure and function of proteins. Proteins are synthesized from instructions coded in the DNA through a multi-step process that includes RNA splicing. Information stored in the DNA of genes is transcribed into immature “pre-messenger RNAs” (pre-mRNAs), pre-mRNAs are then spliced into mature “messenger RNAs” (mRNAs), and finally, mRNAs are translated into proteins. In humans and most other organisms, the splicing process thus ensures proper protein production.
“Targeting the splicing process is a promising strategy for finding new medicines to treat SMA, and possibly other diseases,” said Marcus Rhoades, Ph.D. of the National Institute of General Medical Sciences, which partially supported Krainer’s research. “This work brings us one step closer to that goal.”
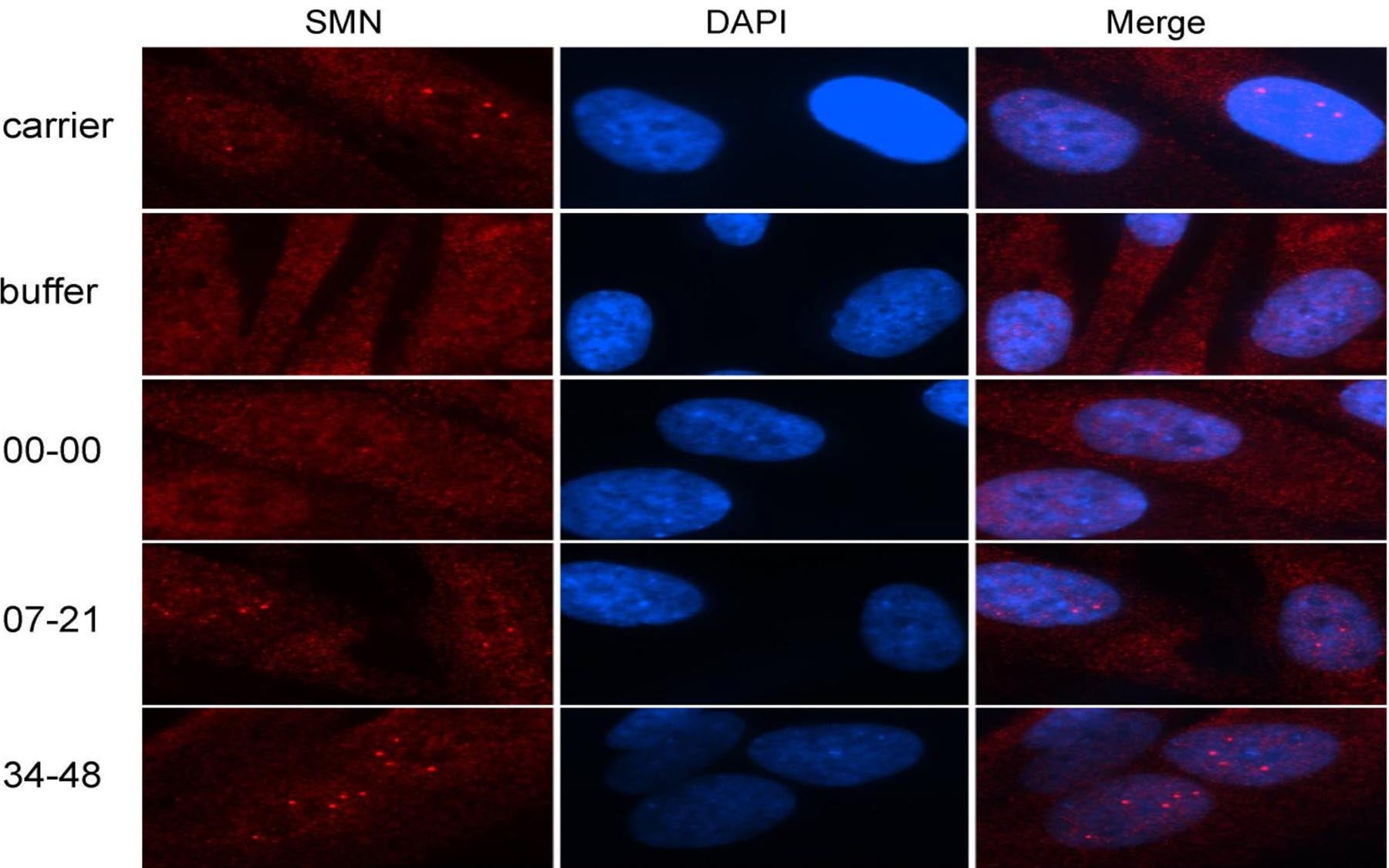
The defect in SMN2 gene expression in SMA patients is at the level of pre-mRNA splicing, such that exon 7 tends to be left out of the mRNA that ultimately makes SMN protein. Several strategies have been pursued to increase the extent of exon 7 inclusion in the splicing of SMN2, for eventual use as therapeutics for SMA. The Krainer team, in collaboration with a team at Ionis Pharmaceuticals, surveyed a large number of antisense oligonucleotides (ASOs) and found that some of these ASOs are able to correct the mRNA splicing defect in cultured cells from SMA patients. These powerful ASOs are identified by the Krainer team as viable for testing in mouse models—the next step in the process of developing new human therapies.
“Families and advocates are very pleased to see the advancement of this antisense technology for the treatment of spinal muscular atrophy. We have high hopes for the success of the next phase of the work”, said Cynthia Joyce, Executive Director of the SMA Foundation, an advocacy group that provides financial support for this project at CSHL.
About Spinal Muscular Atrophy
Spinal muscular atrophy is a genetic disease that causes the degeneration of spinal cord motor neurons and leads to progressive muscle weakness, atrophy and inability to walk or sit, and breathing difficulties. Children afflicted with this disease suffer a premature death due to respiratory failure, generally before reaching two years of age. The SMA Foundation estimates that currently over 50,000 people suffer from SMA in the U.S., Europe and Japan and that a conservative annual market potential for an SMA treatment could exceed $500 million.
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455
Citation
“Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon” – Hua Y, Vickers TA, Baker BF, Bennett CF, Krainer AR (2007) PLoS Biol 5(4): e73. doi:10.1371/journal.pbio.0050073
About the Spinal Muscular Atrophy Foundation
The Spinal Muscular Atrophy Foundation is a nonprofit organization dedicated to accelerating progress towards a treatment and cure for spinal muscular atrophy through targeted funding of clinical research and novel drug development efforts. Since 2003, the Foundation has awarded over $30 million in sponsored research agreements. In addition, the Foundation is committed to raising awareness and generating support for increased research efforts in SMA among the leaders of industry and government. For more information visit www.smafoundation.org or call 646.253.7100.
Principal Investigator

Adrian R. Krainer
Professor
St. Giles Foundation Professor
Cancer Center Program Co-Leader
Ph.D., Harvard University, 1986