Cold Spring Harbor, NY — Acute myeloid leukemia (AML) is an aggressive blood cancer that is currently incurable in 70% of patients. In a bold effort, CSHL scientists are among those identifying and characterizing the molecular mechanisms responsible for this cancer in order to generate potential new therapeutics.
CSHL Assistant Professor Christopher Vakoc, M.D., Ph.D., and colleagues, including the group of Professor Robert Roeder Ph.D. at The Rockefeller University, report the characterization of a protein required for AML in a paper published today in the Proceedings of the National Academy of Sciences.
Screening of DNA packaging proteins calls attention to RNF20
About 5%-10% of AML is characterized by the rearrangement of a gene called MLL (Mixed-Lineage Leukemia). The MLL fusion proteins that result from this rearrangement are potent oncogenes—cancer-causing genes. They are particularly common in infant acute leukemia, which is usually resistant to standard chemotherapy.
An emerging body of evidence implicates problems with chromatin, the packaging material for DNA within the cell nucleus, as a major cause of acute leukemia. Based on this, Vakoc’s laboratory is searching for novel drug targets among chromatin proteins, with the hope of identifying new ways to fight this disease.
“We sifted through a class of proteins that add ‘protein tags’ to chromatin in a search for new therapeutic targets,” says Eric Wang, a former technician in the Vakoc lab who led this study. Wang is currently a doctoral student at Johns Hopkins University. These ‘protein tags’ are called ubiquitin and constitute one of many ways to control which genes are switched ‘ON’ or ‘OFF’ in our cells.
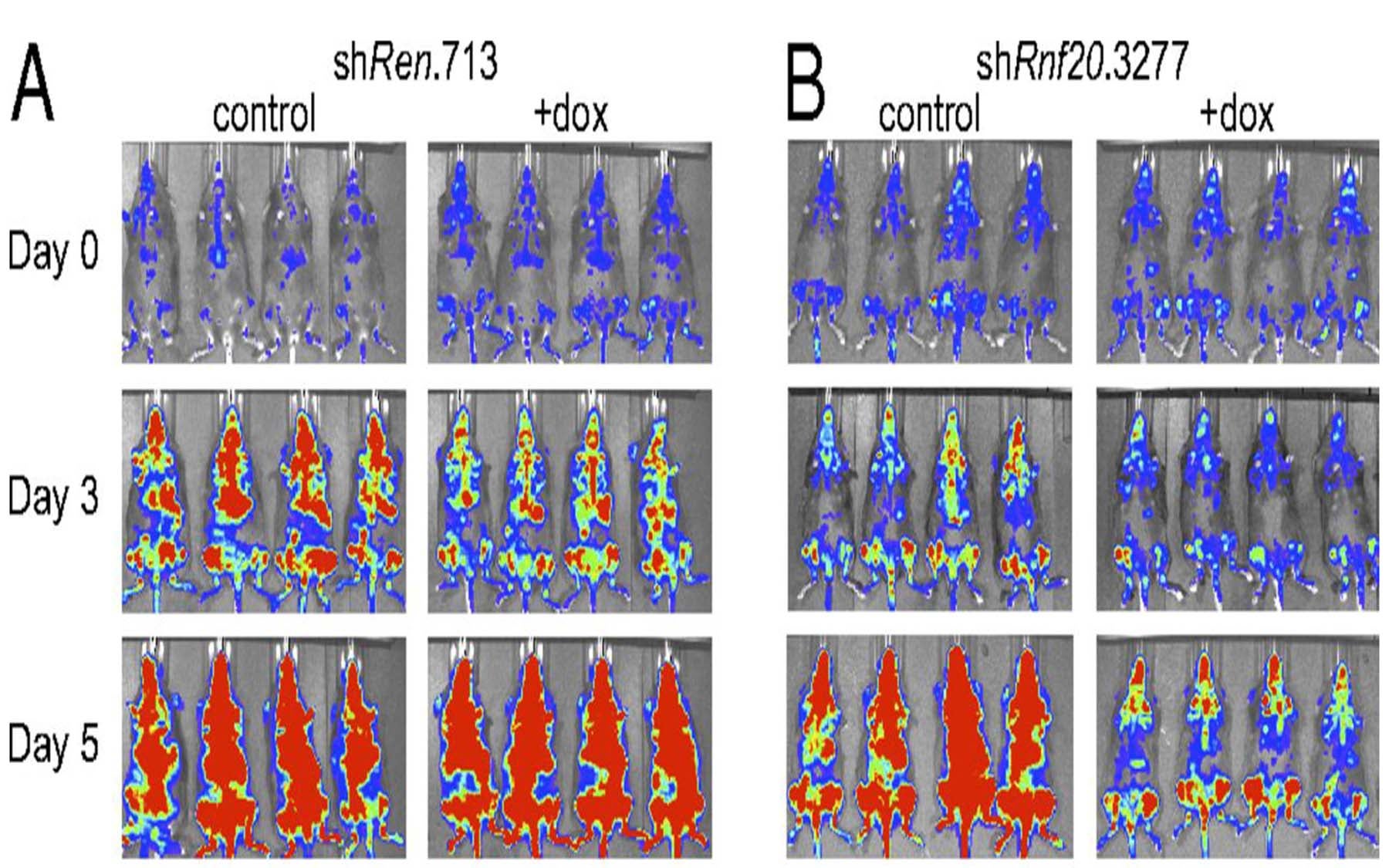
The team identified a protein called RNF20 that attaches ubiquitin tags to chromatin as being essential for the formation of leukemias caused by MLL-rearrangements. When they suppressed the expression of RNF20, the researchers were able to decrease leukemia cell proliferation in living mice, extending their lifespan.
Upon closer examination, the team realized that by targeting RNF20 they prevent MLL-fusion proteins from being able to work. “When we reduce the levels of RNF20 in leukemia cells, they revert back to being more like normal blood cells”, says Shinpei Kawaoka, a postdoc in the Vakoc lab who also was involved in the study.
Vakoc’s team proposes a model in which leukemia cells of the MLL-rearranged subtype come to rely on RNF20 to maintain their gene expression program, implicating it as a potential therapeutic target. Small-molecule drugs that target other proteins involved in regulating ubiquitin tags already exist so it is possible that drugs targeting RNF20 could be developed in the future for the treatment of MLL-rearranged leukemia.
Written by: Edward Brydon, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
The research described in this release was supported by the following grants and funding agencies: A Starr Cancer Consortium Grant I4-A430 (to C.R.V. and R.G.R), the Edward P. Evans Foundation, Martin Sass Foundation, and F. M. Kirby Foundation for research support. C.R.V. is also supported by a Burroughs-Welcome Career Award for Medical Scientists award and National Cancer Institute Cancer Center Support Grant Q:31 Development Funds (Grant CA45508)
Citation
“Histone H2B ubiquitin ligase RNF20 is required for MLL-rearranged leukemia” is published online in the Proceedings of the National Academy of the USA on February 11, 2013. The authors are: Eric Wang, Shinpei Kawaoka, Ming Yu, Junwei Shi, Ting Ni, Wenjing Yang, Jun Zhu, Robert G. Roeder and Christopher R. Vakoc. The paper can be obtained online at doi: 10.1073/pnas.201301045
Principal Investigator

Chris Vakoc
Professor
Alan and Edith Seligson Professor of Cancer Research
Cancer Center Deputy Director of Research
M.D., Ph.D., University of Pennsylvania, 2007