A shift in the pattern of small RNAs occurs in continuously dividing cells as their genomes become epigenetically reprogrammed
Cold Spring Harbor, NY — Much like cancer cells, plant cells grown for a long time outside of their normal milieu, in culture dishes, have highly unstable genomes. Changes in gene activity, or how genes are “expressed,” help cells cope with challenging culture conditions but inadvertently also leave genes prone to misregulation by transposons—bits of DNA that can jump around in the genome, inserting themselves into random genetic locations, often disrupting normal gene function and regulation.
Such genomic chaos, found in cancer and other diseases, is normally prevented by a host of mechanisms that scientists call epigenetic: they modify the expression of genes, although not by causing mutations in the sequence of the genome’s DNA “letters.”
How transposons (sometimes called mobile genetic elements) escape these controls is one of the questions driving the research of Professor Robert Martienssen—a pioneer of plant epigenetics—at Cold Spring Harbor Laboratory (CSHL). By undertaking the ambitious task of mapping the changing epigenetic landscape of continuously dividing plant cells, Martienssen’s team has succeeded in capturing in detail epigenetic alterations and the molecular players that allow transposons to run amok. Their findings are described in a paper published in the December 9th issue of PLoS Biology.
Shifting RNA patterns
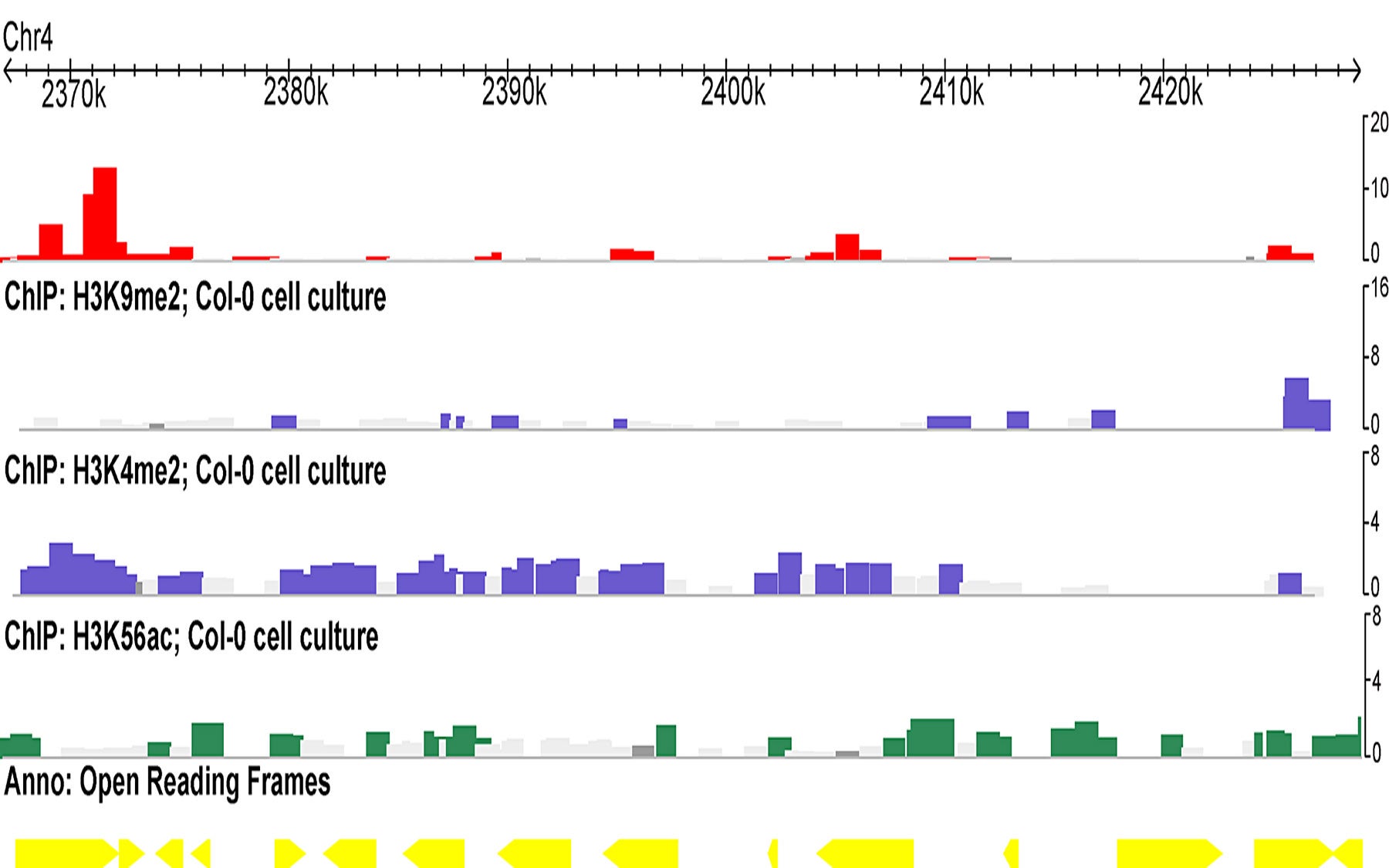
The numerous and diverse transposons present within the plant genome are normally rendered inactive by a series of complicated steps masterminded by small molecules of RNA, called small interfering RNA (siRNA). They perform this feat in a phenomenon known as RNA interference (RNAi). The discovery that modifications of heterochromatin—densely packed, genetically “inactive” regions of DNA—are targeted by RNAi was made by Martienssen’s team in yeast cells and heralded as one of the leading scientific breakthroughs of 2002.
Martienssen’s team now has found that in immortalized cells—cells that are coaxed to grow endlessly in culture dishes—the epigenetic changes resulting in a loss of heterochromatin and transposon “re-activation” are not due to a loss of the proteins that regulate heterochromatin. Instead, they find that the epigenetic modifications are due to a change in the population of siRNAs produced in the continuously dividing cells. When this occurs in the vicinity of genes, this epigenetic control can have important consequences for the organism. According to Martienssen, “our work implicates RNAi in epigenetic chromatin changes that occur in immortalized cells.”
Epigenetic “restructuring” by siRNA
The CSHL researchers find that transposons that have lost heterochromatic marks are no longer associated with siRNA that are 24 nucleotides, or RNA “letters,” in length. Rather, these re-activated transposons become associated with siRNAs that are 21 nucleotides in length. In contrast, the transposons that retain their original heterochromatic marks and therefore remain silent continue to stay associated with 24-nucleotide siRNAs.
The team’s eventual goal is to understand the mechanism responsible for the creation of epialleles—epigenetic variations in gene expression patterns that stem from the creation of particular chromatin states. These predispose particular genes to become active when they shouldn’t be and shut off the activity of genes that are essential. The team’s epigenetic profiling study now implicates siRNA-driven heterochromatin restructuring as a mechanism that might lead to epiallele formation.
Written by: Hema Bashyam, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Citation
“Epigenomic consequences of immortalized plant cell suspension culture” will appear in the December 9th issue of PloS Biology. The full citation is Milos Tanurdzic, Matthew W. Vaughn, Hongmei Jiang, Tae-Jin Lee, R. Keith Slotkin, Byron Sosinski, William F. Thompson, R.W.Doerge, Robert A. Martienssen. The paper is available online at: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.0060302 (doi:10.1371/journal.pbio.0060302)
Principal Investigator

Rob Martienssen
Professor & HHMI Investigator
William J. Matheson Professor
Cancer Center Member
Ph.D., Cambridge University, 1986