Proteins called TAp63 act as genetic modifiers in an illness that produces cleft palate and major deformities of limbs, skin
Cold Spring Harbor, NY — By identifying a protein that acts as a genetic modifier, scientists at Cold Spring Harbor Laboratory (CSHL) have solved the mystery of why some infants are born with a grave syndrome consisting of cleft palate and major deformities of the skin and limbs, while other infants bearing the same predisposing genetic mutation bear little or no sign of the illness, called EEC.
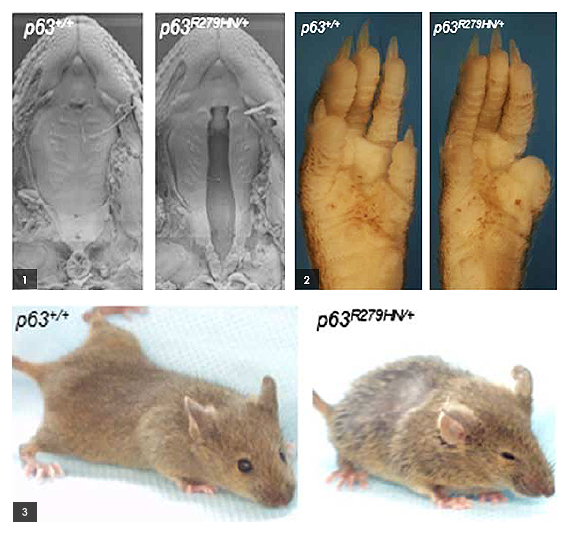
EEC stands for “Ectodactyly, Ectodermal dysplasia, Clefting syndrome.” It is rare in its full-blown form, although individual aspects of the associated pathology, such as cleft palate, are more common.
EEC has a known genetic culprit, a single-“letter” DNA mutation in a gene called p63. This error causes a mutation in the p63 protein that the gene encodes. EEC is autosomal dominant, meaning that only one parent needs to contribute the defective copy of the gene for a child to develop the illness. When one parent carries the mutant gene, each child has a 50% chance of having EEC.
“But the big question is why some children with the mutation have severe birth defects, while others—in some cases, siblings of those affected—who bear the same p63 mutation, are mostly or entirely symptom-free,” says Professor Alea Mills, Ph.D., the CSHL geneticist who led the team that has just solved this mystery.
A complex series of genetic experiments directed by Mills reveals that the presence or absence of one variant type of the p63 protein, called TAp63, determines whether or not a child with the p63 mutation will in fact develop EEC pathology. TAp63 normally protects from the birth defects, and if it is not present, pathology is certain to occur, the team’s experiments showed.
Solving the mystery of variable pathology
In 1999, Mills made the first genetic “knock-out” model for p63, in mice, putting the p63 gene on the map. Mice completely lacking the p63 gene were born with birth defects similar to the severest symptoms that characterize EEC in humans. Now, with a grant from The March of Dimes Foundation, Mills’ team is the first to make a “knock-in” mouse model of EEC in which they replaced the normal p63 gene with a version bearing the single-letter mutation that causes EEC in people.
“We’ve made the very first mouse model of human EEC syndrome,” notes Emma Vernersson Lindahl, Ph.D., a postdoctoral researcher and lead author of a paper appearing today in the American Journal of Medical Genetics that announces the team’s results. “These mice, like babies born with EEC, showed a range of birth defects, fully recapitulating the range of defects that one sees in the human syndrome,” she says.
To solve the mystery of variable pathology, the CSHL team tested the idea that the p63 protein itself controlled or “modified” EEC’s manifestation in different individuals. The scenario they tested and which proved successful was this: mice with the EEC-causing p63 mutation were crossed to mice engineered to lack TAp63, one of the two major classes of p63 proteins. Those with both genetic changes consistently had features of EEC.
Most genes generate instructions for manufacturing proteins; precisely how the gene is turned on or its message edited affects which versions of structurally distinct proteins are manufactured. All healthy people generate both major classes of p63 proteins. TAp63 proved to be the class of p63 protein that modifies EEC features. Mice lacking TAp63 did not have any pathology, which means that TAp63 loss alone is not responsible for the syndrome. But when mice lacking TAp63 also possess the EEC-causing p63 gene mutation, pathology always occurs.
This work suggests that levels of the TAp63 protein determine whether children that have inherited one copy of the EEC-causing mutation from one of their parents are born with birth defects. Mills speculates that when levels of TAp63 drop beneath a certain threshold, it is no longer protective, opening the way to pathology.
“The only way you can have the EEC mutation and be normal, or have slight symptoms of the illness such as a bit of webbing between two toes, is to have robust amounts of TAp63 protein in cells when and where it is needed, during development,” says Mills.
She hopes that her team’s discovery that TAp63 affects the presence of birth defects will encourage doctors treating children with EEC to compare those only mildly affected with siblings or other children who have a severe form of the disease. “It will be important to sequence DNA from these children and compare the results. What’s different? If we find differences, we have nailed it. If we find that the sequences are exactly the same, then we might look at several factors regulating gene expression for evidence of how TAp63 is expressed differently in each group.”
Written by: Peter Tarr, Senior Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
The research described above was made possible by grants from The March of Dimes Birth Defects Foundation; the American Cancer Society; the Swedish Research Council; and the Lauri Strauss Leukemia Foundation.
Citation
“An Allelic Series of Trp63 Mutations Defines TAp63 as a Modifier of EEC Syndrome” appears June 14, 2013 in the American Journal of Medical Genetics. The authors are: Emma Vernersson Lindahl, Elvin L. Garcia, and Alea A. Mills. The paper can obtained using the DOI 10.1002/ajmg.a.36074
Principal Investigator

Alea A. Mills
Professor
Cancer Center Member
Ph.D., University of California, Irvine, 1997
