Cold Spring Harbor Laboratory Associate Professor and Cancer Center member Tobias Janowitz led a COVID-19 clinical trial with Northwell Health in 2021. When he and Clinical Fellow Hassal Lee reviewed the data, a surprising trend emerged. “The patient roster was very diverse,” Janowitz explains. “We’d made no deliberate effort toward that other than conducting the trial remotely.”
When it comes to cancer trials, many variables impact patient participation. One measurable factor is distance. On average, people are less likely to sign up for trials more than 30 minutes away. Karen Winkfield, Executive Director of the Meharry-Vanderbilt Alliance and member of the National Cancer Advisory Board, says:
“The biggest reason patients don’t enroll is they’re not asked. They aren’t asked because there may not be clinical trials close to them.”
Now, Janowitz and Lee have developed a new approach that may help clinics recruit patients from more communities. Using data from the U.S. Census Bureau, National Trial Registry, and other publicly funded organizations, the team created population maps for areas around highly active cancer trial sites. They found that high-volume sites are often in affluent neighborhoods with less diverse populations.
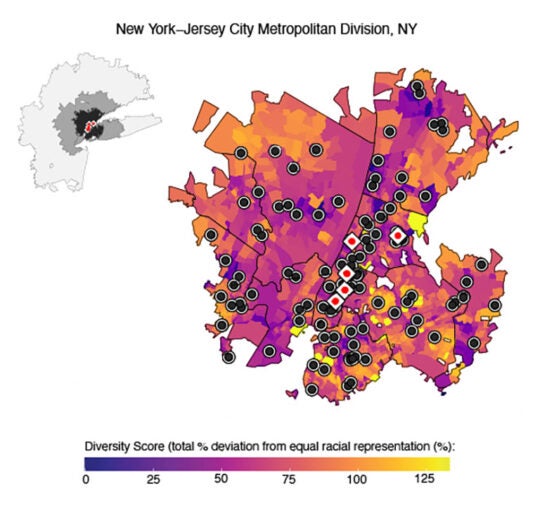
“Clinical trials should be accessible to all,” Janowitz says. “Currently, 78 sites host about 94% of all U.S. cancer trials. We offer a new approach for these and other sites to use available data in designing more equitable clinical trials.”
With their new tool, the team analyzed the sites’ neighboring areas. They then identified nearby hospitals serving more diverse communities. Collaborating with these potential satellite sites could help expand patient recruitment efforts.
“For example, if you wanted to diversify the population for a clinical trial, you could use our tool to generate maps with whatever demographics you want,” Lee explains. “It’s transferable to any U.S. area as long as you have Census data.”
Janowitz and his team are now working to expand their analysis to other population segments and trials unrelated to cancer. They hope their new approach will help streamline clinical trials. Winkfield, who co-authored the study, says:
“We can do a much better job of providing access to clinical trials in the communities where it matters most. If we’re able to use this tool to make real collaborations with community health centers and hospitals that are interested, this could be a game changer.”
The next step is to measure potential real-world impact. Janowitz and Lee suggest applying their approach to design new cancer trials. If they’re right, the trials will be more readily available to all.
Written by: Nick Wurm, Communications Specialist | wurm@cshl.edu | 516-367-5940
Citation
Lee, H., et al., “Analysis and Optimization of Equitable U.S. Cancer Clinical Trial Center Access by Travel Time”, JAMA Oncology, March 21, 2024. DOI: 10.1001/jamaoncol.2023.7314
Principal Investigator

Tobias Janowitz
Associate Professor
Cancer Center Program Co-Leader
M.D., Ph.D., University of Cambridge, UK, 2007
