Sometimes sugar isn’t so sweet. Sometimes, it’s downright deadly. Glycation occurs when an excess of sugars called reducing sugars attach to important proteins in the body. The process has been linked to diabetes and obesity. It’s usually something to avoid, and the body relies on the FN3K kinase to break glycation down. But cancer flips everything on its head.
In certain types of cancer, glycation prevents tumors from taking advantage of a protein that helps them grow and spread. In those cases, Cold Spring Harbor Laboratory (CSHL) Professor and HHMI Investigator Leemor Joshua-Tor says, you don’t want FN3K involved. She explains:
“The tumor needs a lot of sugar to grow. Because of that, the sugar gets attached spontaneously onto this protein and dampens it. It’s like stuffing something in its mouth. The tumor can’t proliferate and grow.”
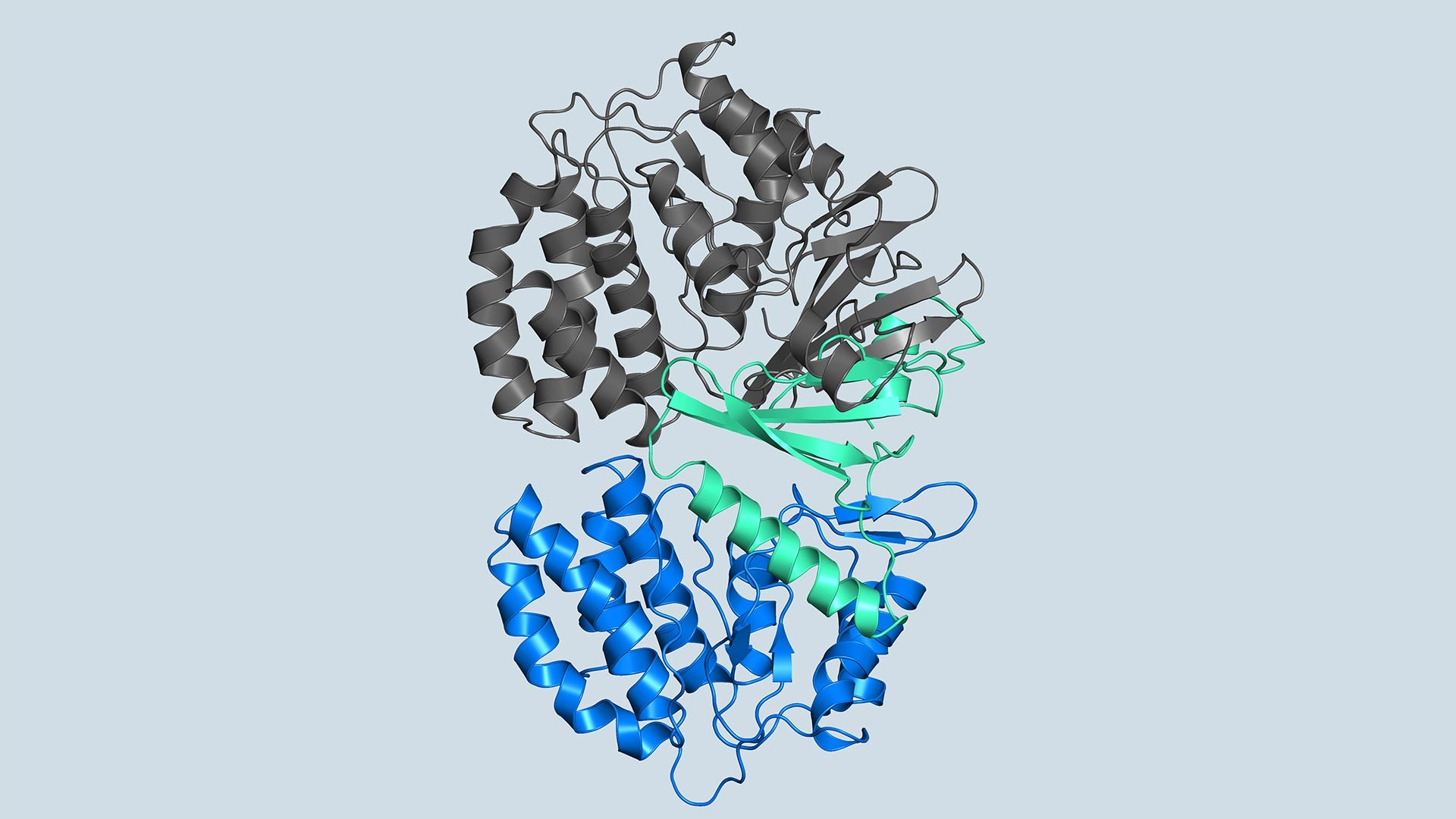
FN3K’s role in breaking down glycation has been known for decades. But the structure of the kinase, needed for drug development, has remained a mystery until now. Joshua-Tor, CSHL postdoc Ankur Garg, and their team have revealed 3D structures of human FN3K captured in different states for the first time. These structures could point the way to new cancer therapies, offering hope for patients suffering from the deadly disease.
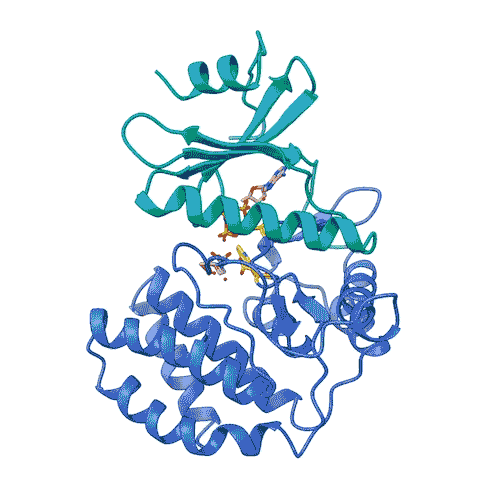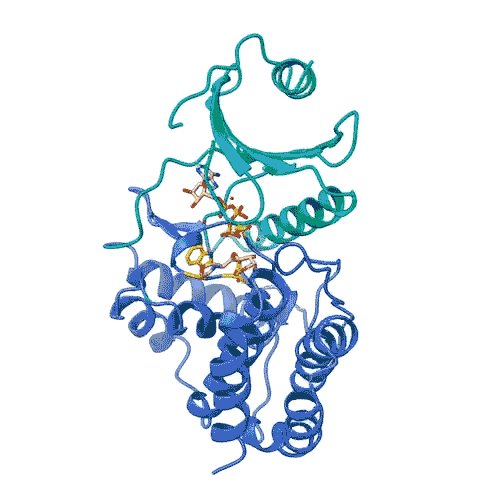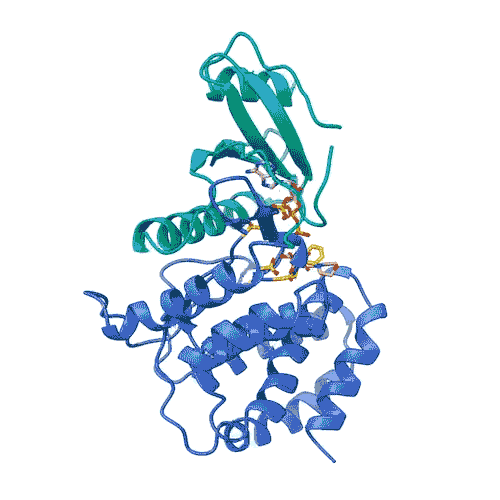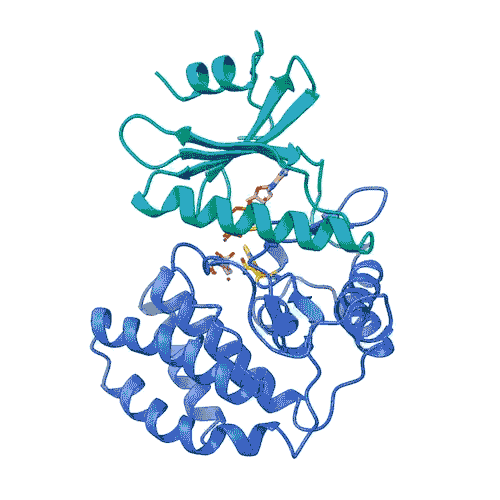
“Kinases are common in our cells,” Joshua-Tor says. “So, in order to target only FN3K, and not the other kinases we need, we have to know exactly what it looks like. This gives us a precise model.”
The model enabled the team to lay out the entire process by which FN3K breaks down glycation. They found that, unlike most kinases, human FN3K contains an amino acid called tryptophan. Yes, that’s the same tryptophan that knocks you out after a big meal. When the team removed it from FN3K, the kinase became inactive. And when they changed it to a different amino acid, it transformed FN3K into a “super-enzyme,” Garg says.
“Controlling one tiny amino acid can control the whole enzyme,” he explains. “This is important for designing specific drugs to target only FN3K.”
Collaborators at Memorial Sloan Kettering Cancer Center are now using the lab’s new structures to do just that. Future clinical applications are still far away. However, Joshua-Tor says the first step is to identify existing small molecules that bind to FN3K.
“It’s really remarkable when you think about it,” she says. “These enzymes are so fine-tuned that everything has to be in the right state at the right time. It would be great if we could exploit that with an initial drug or molecule and go forward from there.”
Written by: Nick Wurm, Communications Specialist | wurm@cshl.edu | 516-367-5940
Funding
The Starr Foundation, National Institutes of Health, Howard Hughes Medical Institute
Citation
Garg, A., et al., “The molecular basis of Human FN3K mediated phosphorylation of glycated substrates”, Nature Communications, January 22, 2025. DOI: 10.1038/s41467-025-56207-z
Core Facilites
Principal Investigator

Leemor Joshua-Tor
Professor, Director of Research & HHMI Investigator
W.M. Keck Professor of Structural Biology
Cancer Center Member
Ph.D., The Weizmann Institute of Science, 1991