Cold Spring Harbor, NY — Structural biologists at Cold Spring Harbor Laboratory (CSHL) and collaborators at Emory University have obtained important scientific results likely to advance efforts to develop new drugs targeting NMDA receptors in the brain.
NMDA (N-methyl D-aspartate) receptors are found on the surface of many nerve cells and are involved in signaling that is essential in basic brain functions including learning and memory formation. Problems with their function have been implicated in depression, schizophrenia, Alzheimer’s and Parkinson’s diseases, as well as brain damage caused by stroke.
Normally, NMDA receptors are activated by glutamate, the most common neurotransmitter of excitatory cell-to-cell messages in the brain.
Overactivation of NMDA receptors is a known cause of nerve-cell toxicity. Thus, drug developers have long sought compounds that can selectively block or antagonize NMDA receptors, while not affecting other types of glutamate receptors in the brain, whose function is essential. However, a basic question—how those compounds bind and antagonize NMDA receptors—has not been understood at the molecular level.
Over a period of years, CSHL Associate Professor Hiro Furukawa and colleagues have taken a step-by-step approach to learn about the precise shape of various subunits of the complex NMDA receptor protein, and demonstrating the relationship between different versions of the receptor’s shape and its function. Since the subunits have different biological roles, they have to be specifically targeted by drug compounds to obtain specific effects.
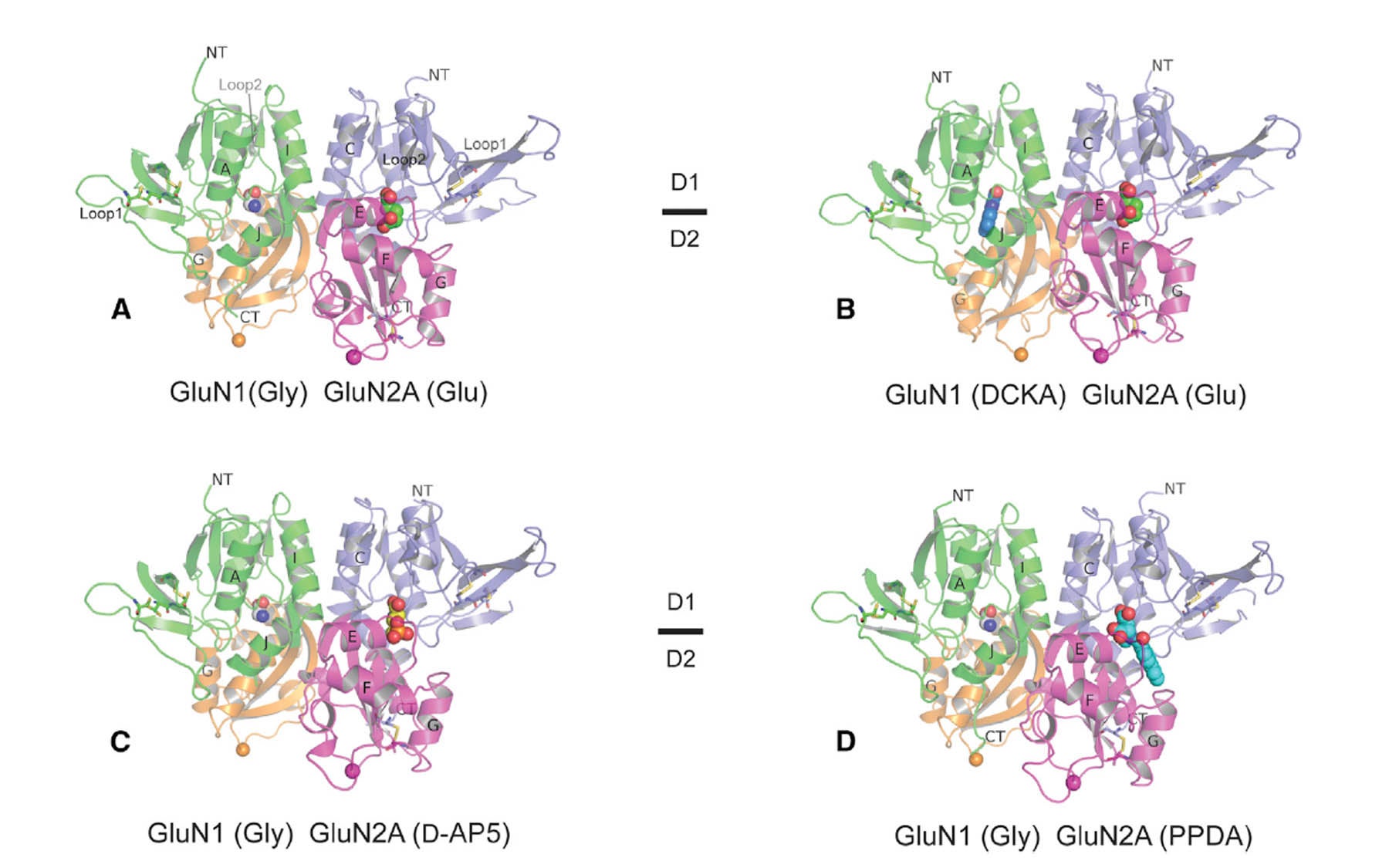
Furukawa’s team has used a technique called x-ray crystallography to map various domains of the protein while it is bound to different chemical compounds, or antagonists, that down-regulate its function. Today in the journal Neuron they publish the first crystal structures of two NMDA receptor subunits (called GluN1 and GluN2A) in complex with four different compounds known to have the capacity to inhibit, or antagonize, NMDA receptor function.
Showing this two-unit ligand binding domain (LBD) in complex with NMDA antagonists—potential drugs—reveals that each antagonist has a distinctive mode of binding the LBD. In essence, the “docking port” is held open, but to a different extent when different antagonists are bound. The study also reveals an element in the antagonist binding site that is only present in GluN2A subunit, but not in the others. This previously hidden information, says Furukawa, is critical: “It indicates different strategies to develop therapeutic compounds—ones that bind to a certain type of NMDA receptors very specifically. Being able to target specific subtypes of the receptor is of enormous interest and has great therapeutic potential in a range of illnesses and injuries affecting the brain.”
Written by: Peter Tarr, Senior Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
This research was supported by grants from the National Institutes of Health, the Robertson Research Fund of Cold Spring Harbor Laboratory and Japan Promotion of Science.
Citation
“Structural Insights into Competitive Antagonism in NMDA Receptors” appears online ahead of print in Neuron on January 22, 2014. The authors are: Annie Jespersen, Nami Tajima, Gabriela Fernandez-Cuervo, Ethel C. Garnier-Amblard and Hiro Furukawa. The paper can be obtained at: http://www.sciencedirect.com/science/article/pii/S0896627313011306.
Principal Investigator

Hiro Furukawa
Professor
Cancer Center Member
Ph.D., The University of Tokyo, 2001