As a graduate student at The Ohio State University, Jeremy C. Borniger wondered why so many cancer patients had sleep disorders. That question would help set the stage for a completely new field of research now known as cancer neuroscience. Today, Borniger is an assistant professor at Cold Spring Harbor Laboratory. We visited his lab for an inside scoop on this up-and-coming field. It turns out Borniger’s work may also have implications for treating diseases beyond cancer. Which ones? Try all of them.
[Questions and answers have been edited for clarity.]
What is the focus of your lab?
A mishmash of cancer and neuroscience. The concept we’re working on takes cancer as a new developing organ in the body. We’re focusing on how the host system, whether that’s your immune system, metabolic system, or nervous system, responds to this growing malignancy. How does a developing tumor disrupt normal physiology? How does the brain sense those changes in the body? And then how does it respond?
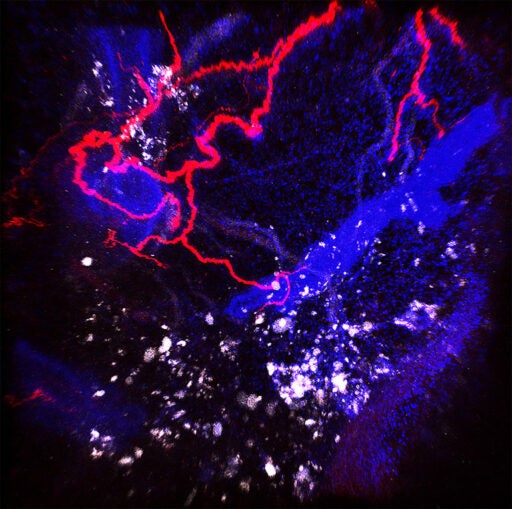
What do nerves have to do with cancer?
Nerves go to essentially every tissue in your body. And that includes developing tumors. In preclinical studies where you use a drug to get rid of nerves in the tumor, those tumors don’t grow anymore. And if we manipulate the signals those nerves provide to tumors, we can also tune tumor growth up or down. We’re just starting to figure out what are the knobs and dials in that system that we can mess with. But essentially I think we have a missing piece that we’re starting to incorporate now into our conversation around eliminating cancer.
Why do tumors care about nerves?
Tumor cells recruit nerves. One reason is so they can escape. They sort of hide out in nerves. Recent studies have shown nerves can provide metabolic support to tumor cells, especially in nutrient-poor conditions. So, cancer cells steal nutrients from nerves.
Another thing is that certain nerves release neurotransmitters that restrict the blood vessels in the tumor a little bit. That causes hypoxia—lack of oxygen in the middle of this tumor—and that prevents immune cells from getting into the tumor. If immune cells can’t get in, they can’t eliminate the tumor.
What’s the potential of this new cancer neuroscience field?
Because the nervous system is electrically excitable, the approaches we can use to manipulate nerves have an additional level of control besides injecting a drug. We can use the natural wiring of the nervous system. I think a combination of drug approaches with something like bioelectronic approaches will hit that sweet spot of how can we manipulate the nerves but also set them into the right mode to eliminate cancer.
Bioelectronic approaches?
We can actually use electrical devices to control the nervous system. Bioelectronic medicine is trying to basically strap a little electrode to one or more nerves in your body. Then, when there’s aberrant signaling going on, we can either stop that or elicit good signaling from those nerves. It’s similar to how pacemakers work, although significantly more complicated.
Take a look inside Borniger’s lab as he discusses the new field of cancer neuroscience and its therapeutic potential.
How do you figure out what bioelectronic “dosage” someone gets?
Because nerves communicate with electricity, the amplitude and frequency of those signals are very important. Say a fast signal would indicate to your nerve that this is a dangerous situation, and if you have a lower-frequency signal associated with this, it isn’t a bad situation. We need to first figure out what signals cancer elicits in the nerves, and then what those signals mean. We need to figure out how can we read that signal as it travels along the wire. Once we know that, we can inject signal into the nerves using these electrical devices to tell the tumor what we want.
How far are we from bioelectronic medicine hitting cancer clinics?
It’s at the startup “Silicon Valley” level now. But those things will grow quickly as technology keeps improving. Then, of course, regulatory problems go along with, you know, selectively electrocuting certain parts of the body, which is already something we do with deep brain stimulation devices, pacemakers, and these sorts of things. There are companies using bioelectronic medicine in the clinic to treat various of conditions, including rheumatoid arthritis, depression, and treatment-resistant epilepsy.
Why is this a new field?
The question I get a lot is: why haven’t we been looking at this? I think part of it is that people who work on nerves didn’t talk to people who work on cancer until relatively recently. Cancer people are siloed into cancer labs, and neuroscience people into neuroscience labs. Now, we need to expand and see what are the limits of the nervous system in cancer processes.
How did you get into this field?
I came to it from an outside-in perspective. My lab in graduate school was focused on the neuroscience of sleep and circadian rhythms—totally unrelated to cancer. But I went to a couple of different talks where doctors and nurses described problems cancer patients complain about, like sleep disruption, cognitive fog, and changes in appetite.
I thought immediately, ‘Well, this is a neuro problem because we experience sleep with our brain.’ I tried to find a paper that had done these experiments and was surprised that no one had done them before. So that was the idea that we went with. And it turns out that, yes, the tumor itself, without any extraneous influences like diet, age, or sex, can powerfully influence the arousal state.
So sleep disruption isn’t just a side-effect of cancer treatments?
Studies have shown that patients with poor sleep and chronic fatigue tend to do worse than those with adequate sleep. I think what has always been a tacit assumption is that, of course, patients with cancer have bad sleep. They’re stressed about having cancer. They’re taking these toxic therapies that mess up a lot of things, including sleep.
Right, so how does a tumor throw off your sleep cycle?
By altering signals in the body that the brain normally detects. Normally, if you’re hungry, for instance, that’s associated with changes in your endocrine system, and neurons in your brain sense these changes. If certain levels are too low or high, these neurons tell you to wake up and find food. Your brain will actually prevent you from sleeping by turning on these arousal systems to reestablish normal energy balance.
We found that the tumor, which is nowhere near the brain, was causing the host system to send out these aberrant signals. Then the brain would interpret that as saying, “Oh, you’re hungry so wake up and find food.” The effect is your sleep is all messed up. When we blocked the activity of these neurons in mice, the mice with cancer slept normally. And that was associated with benefits in systemic health.
Does the potential of bioelectric medicine go beyond cancer?
It is just a matter of time to figure out the right combination of pharmacological and bioelectronic approaches to target many different malignancies and diseases. Cancer’s not the only disease of neural involvement. I think many of the tools we’re developing for cancer will also apply to other problems, especially inflammatory disorders.
Do nerves affect all diseases?
I forget who said, “No cell in your body is more than 10 microns away from a blood vessel.” That’s essentially the same for a nerve. Basically, everywhere blood goes, there are nerves nearby. So, if you imagine any sort of problem, literally anywhere, nerves are going to be involved or detecting what’s going on.
Are there any examples of this brain-body connection affecting our daily lives?
If you’ve ever been really stressed out—say an exam is coming up or you have a big life event. And then as you’ve been getting stressed, all of a sudden you get sick. This is a common thing many people have experienced, and this is because your nervous system, which is where you’re experiencing that stress, controls your immune system. So, this stressful experience can be relayed down to your body and tell your immune system to dampen its response. That allows latent infections and viruses to spring up at the worst time.
There’s a famous study of medical students, where they collected blood leading up to a major exam. As stress builds up, you can see the titers of herpes virus in their blood go up and up because the immune system is getting worse and worse at keeping that at bay. Then, immediately after the exam, when stress levels go down, you can see the virus levels go way down. It’s really this interesting cross-talk between the brain and immune system that we can start to harness for virtually any disease.
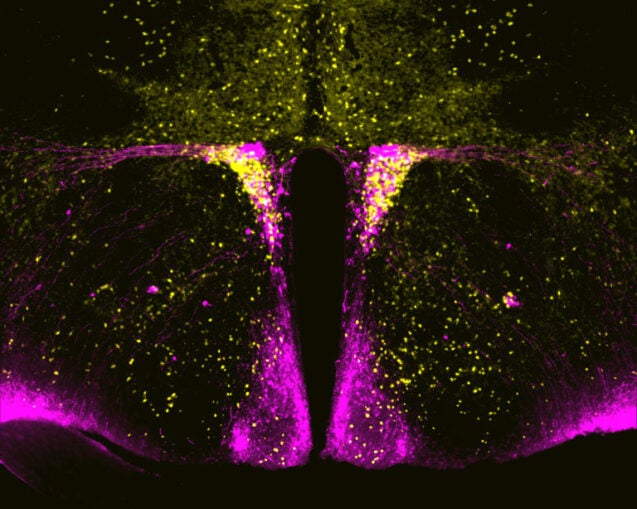
But we still don’t know which nerves make us feel sick.
There’s a big push in the neuroscience or whole-body physiology community to figure out how something in your body can influence this complicated change in your brain.
If you’ve ever felt ill, you’re not operating the same way. You’re not going for a run or going to the store and doing your normal thing. Your brain sets your body into a different state: rest, relax, and recover. That’s obvious to most people, but we don’t know how that works, which is surprising because across virtually all illness, being tired is a common phenomenon. There’s a separation between being sick and feeling sick, and we need to figure out how being sick causes you to actually feel sick.
Once we figure out which nerves make us “feel” sick, what then?
That’s a really exciting area because it turns out, at least in mouse models, the neurons responsible for feeling sick can influence the body in a way that can actually fight the sickness. All diseases have some relationship with nerves and that’s a field that hasn’t been explored much. There might be potential, new avenues for therapeutics.
The future of cancer therapy looks bright thanks to pioneers like neuroscientist Jeremy Borniger. By delving deeper into brain-body connections, he hopes to help bring new treatment options from the lab to the clinic. While still in its early stages, bioelectronic medicine is already sending shockwaves through cancer neuroscience and beyond. How far will those waves reach in the fight against disease? We’ll soon find out.
Written by: Luis Sandoval, Communications Specialist | sandova@cshl.edu | 516-367-6826
About

Jeremy C. Borniger
Assistant Professor
Cancer Center Member
Ph.D., Ohio State University, 2017

