BasePairs podcast
One in six people suffers from a mental disorder, and yet, compared to cancer and infectious disease, neuropsychiatric treatment options have barely improved in decades! Why is that?
In this episode of Base Pairs, we talk to Stanford Professor Robert Malenka about the limitations that classic business practices place on modern drug development. Looking for another perspective, we also chat with Dr. Raymond Hill, a former Executive Director, Licensing and External Research, Europe, for the pharmaceutical giant Merck. He describes some ways to make the pursuit of drug targets more attractive to industry. Lastly, we hear from CSHL Professor Tony Zador, whose uniquely genetic approach to mapping the brain may help revolutionize how neuropsychiatric drug discovery is done.
BS: And I’m Brian.
AA: And this is BasePairs, the podcast about the power of genetic information.
BS: So put your thinking caps on because today, I wanted to start with the discussion about thinking or what can go wrong with thinking.
AA: Yes, it is a special 2016 Election Edition of BasePairs. Okay, not really. We’re actually going to talk about neuropsychiatric disorders today. Those familiar but still puzzling illnesses like depressions, schizophrenia, and bipolar-disorder that affect 1 in 6 people across the globe.
BS: That’s according to the World Health Organization, and it’s stunning statistic. In the U.S. alone, things get even worse. The NIMH reports-
AA: That’s the United States National Institute for Mental Health.
BS: According to them, of all the potential years lost to illness, disability, or death among U.S. citizens, 20% were effected by a neuropsychiatric disorder. It’s the largest percentage, topping heart problems, cancer, injuries, even respiratory disease.
AA: Meaning mental problems are the biggest foe to quality of life for U.S. citizens, and those stats are similar in Europe.
BS: And here’s the kicker. Despite being such an impediment to public well-being, the number of distinct treatment options for the mentally-ill have barely increased from where they were decades ago.
RM: There really hasn’t been a drug with a truly novel mechanism of action for treatments of any brain disorder, especially in psychiatry, in probably 50 or 60 years.
BS: That’s Rob Malenka, a professor of Psychiatry and Behavioral Science at Stanford University. I recently spoke with him about what many call the drought of neuropsychiatric drug discovery, and his frustration is palpable.
RM: I think it’s absurd because while the medications we use in psychiatry and in neurology are often quite effective and helpful, there are still millions of patients who are suffering, who don’t get adequate treatment, and so at some point, you have to say, “Well, maybe we should change the strategy,” but I want to be right upfront, I am a founder of a biotech called Circuit Therapeutics because I became so frustrated with the lack of innovation in big corporation, big pharma, and I will talk a little bit about why I think that is.
BS: Rob, with his academic background, is one side to an important coin. I wanted a perspective from someone from pharma as well, wondering if they had anything different to say, so I reached out to Dr. Raymond Hill.
So Dr. Hill, can I call you Ray?
RH: Oh, Ray is fine. I’m a pharmacologist. I’ve worked in the pharmaceutical industry for nearly 30 years. I had the honor to be President of the Pharmacological Society about 3 years ago now.
AA: If you haven’t guessed from the accent, that’s the British Pharmacological Society, but while Ray spoke to us from London, where he enjoys his retirement as a visiting professor at Imperial College London, his knowledge of drug discovery spans years and continents alike.
RH: If you think back to the 1950’s when the first drugs that were useful in treating psychiatric disease were discovered, that was a result of brilliant empirical observations by scientists and clinicians who were working with patients and realized that drugs that were being given to treat one condition, in fact, benefited the psychiatric condition that the patient actually suffered from.
BS: Think about what Ray is saying here. He’s saying that some of the first neuropsychiatric drugs discovered over 60 years ago are still used today. Drugs like lithium for bipolar disorder and the first generation of anti-psychotic medicines, and the thing is-
AA: They were discovered accidentally. It’s wild.
BS: It is. At the time, you can see how this was a breakthrough. With hardly any understanding of the brain, nevermind mental illness, even having a little understood treatment option was huge. These discoveries became the stepping stones for neuropsychiatric pharmacology. With experts trying to reverse engineer and improve these serendipitous solutions.
AA: That seems like a good plan, but I think I see the problem already. An approach taken decades ago may not be the best option today. Science as we’ve learned time and time again is always changing.
RM: People have spent decades trying to figure out the true therapeutic mechanism of action of the tricyclic antidepressants, which have been around for 50 years or the drugs like Prozac, and the problem with that approach is people have been working on that for 50 years.
BS: That’s Rob again, and as you can probably tell, he thinks dwelling on the same drugs, the same accidental solutions is what’s holding progress back in the long run.
RM: And if they keep doing what they’re doing, and I’m using the generic “they” for Big Pharma, they all claim they’re doing new stuff and innovative stuff, but the truth is they really aren’t. They’re actually very conservative. Many of the Pharma are dominated by the business development people who are saying, “We need a new drug for this indication or that indication within 10 years that’s gonna be a blockbuster.” I hope I’m making sense here.
AA: He’s making a lot of sense actually. New discovery is always slow going, and with an organ as complex as the human brain, to ask a pharmaceutical company to put a decade or more into anything other than the drugs and approaches they’re familiar with is asking them to gamble.
RH: It’s a huge risk, and there are many more addressable things that one can do in metabolic disease or cancer that a lot of companies are finding more attractive, and I think it’s no accident that diseases where there are addressable targets but where there is still a large clinical need, such as cancer and diabetes, are the sorts of areas where most major companies are concentrating their resources.
BS: And here’s the point where both Ray and Rob’s explanations for the lack of neuropsychiatric drug discovery, the reasons we have seen so few new drugs for mental illness over the last five decades. They begin to almost seamlessly blend.
RM: I wanna be fair. I think there are many, really great scientists working in drug discovery for the brain at many different pharma, both large and small, who are dedicated, who are creative, who really want to have an impact on human health and are doing their work for all the right reasons. However, they face many political challenges working in a corporate environment. That includes competing for resources with other therapeutic areas that are very important, like oncology, but relatively speaking, relatively, are much easier to understand and develop drugs for.
RH: I mean if you look at the other end of the spectrum. The way we’re approaching cancer now is, in a very mechanistic way, based on a fairer understanding of the molecular biology of particular [Chuma 00:08:46] types. In the case of mere psychiatry, we know almost nothing. I mean, if you look at a disorder like Schizophrenia, if you [troll 00:08:56] the literature, you could come up with as many as a thousand candidate genes that might have something to do with the disease, but there’s no clear correlation with an easily addressable target that would allow scientists in the pharmaceutical industry to start a new drug discovery project.
BS: You see the problem? For decades, trying to find a new drug target for a mental disorder has been like trying to plan a meal without knowing what you have in the pantry or going for a hike without knowing the trail. It can be done, but it’s going to take time and will be very risky.
RM: You know, what I tell my lab all the time is, “You know, the brain is complex.” But that doesn’t mean we should be nihilistic and give up. I think there are approaches that are more valuable than others.
AA: Maybe, and call me out if I’m wrong here, but maybe it’s time to get a look at that pantry to get to know those trails.
BS: Maybe it is.
TZ: My own research, until about five or six years ago, focused largely on studying the behavior of rats performing auditory decisions and trying to tease apart the circuits that are responsible for these auditory decisions. And in the course of that work, what I realized was that we were really stymied by our lack of understanding of the actual circuitry.
AA: That’s Dr. Anthony Zador, a professor whose lab is based right here at CSHL.
BS: We call ’em Tony.
TZ: So we had great tools for manipulating classes of neurons for asking whether one collection of neurons might play one role in a behavior and another class of neurons might play another roll, but what we didn’t really know were what the classes were, who their partners were, who these neurons talked to, where they sent their information.
AA: If you follow neuroscience at all, you probably know where this conversation is heading. To really move forward, people like Tony figured they were going to need a map of the brain, a concept popularly called the Connectome.
TZ: Part of that started to change, maybe five years ago, when the Allen Brain Institute decided to systematically go through and map the long range connections of a mouse brain, and they did that by injecting a tracer in each of about a thousand different regions of the brain of a thousand different mice and then following where that tracer went, and that has been an incredibly useful resource for my lab and many other labs to have an overall view of the architecture of a mouse brain.
However-
AA: However, this really seems like just a start. General regions of the brain? Doesn’t sound as detailed as I’d have hoped.
BS: That’s right. This visual approach, while informative, can’t tell you much at all about what individual neurons are doing inside those general regions.
TZ: One way to think about it is as though you’re going to an airport, and you see the counters for a dozen different airlines, and that airport flies pretty much everywhere in the world, but if you go to a particular airline, that particular airline will only serve probably a subset of the locations, so if you go to Georgian Airlines, you’ll be able to fly to the Middle East but probably not to San Diego, and if you go to Icelandic Airlines, you might not be able to find a flight to Peru.
So each of those little airlines corresponds to a neuron in this analogy. Overall, the airport will probably serve many or most locations in the world, but the individual neurons don’t send their information everywhere.
BS: It stands to reason that to catch the right flight, you really need to determine where each airline is sending its planes. So that’s what Tony set out to do, to match each individual neuron’s connections.
AA: But wait, Brian, it’s been estimated that the average human brain has anywhere between 80 and 100 billion neurons, a number so immense that no one has even been able to come up with a more accurate figure yet. So you’re telling me that Tony hopes to trace all of them?
BS: Remember how we are a podcast about genetic information? We’ve gotten pretty deep in this neuroscience stuff, so I thought I’d remind you. Human chromosomes range in size from about 50 million to 300 million base pairs. Imagine the immense number of possibilities that can come from that, and yet-
AA: The Human Genome Project.
BS: The Human Genome was published in its entirety on April 14, 2003, and since then, genome sequencing has gotten much faster and much, much cheaper.
TZ: So the idea that I had was that, if we stop thinking about neuroanatomy solely as problem of microscopy, if we stop thinking about maps as a map that you can visualize, if we can convert the problem into a problem of high throughput DNA sequencing, then we’d be able to look at the individual connections of thousands and potentially hundreds of thousands or millions of neurons at once. And so when I had that idea, about six years ago now and have been working on it pretty doggedly since, and recently, it’s started to pay off.
BS: Taking cues from genome sequencing, Tony and his lab recently mapped about 50,000 neurons in the central cortex of a mouse, and it only took one mouse and a couple of days to complete.
TZ: So we developed a technique called [MAPSeq 00:15:15]. The “Seq” stands for sequencing, and the map coincidentally is an acronym for Multiplexed Analysis of Projections.
AA: Apparently, one of the keys when developing a new technique is to give it a catchy name.
TZ: So in this technique, what we do is we label every neuron with a random sequence of DNA. We call it a barcode, and the idea is that these random sequences, there’s so many of them that the chances that two neurons get the same random barcode are infinitesimally small, so each neuron now has a unique identifier.
AA: From what I’ve heard, these barcodes are a lot like what you would see at a supermarket. What makes one can of soup different than another, a single stream of numbers, and yet, it let’s Stop & Shop know that [Joe-Shmoe 00:16:18] bought a can of creamy mushroom and not chicken noodle.
BS: Maybe he’s making green bean casserole.
AA: Science, Brian. We were talking about science.
BS: Gastronomy is a science.
AA: Neuroscience. Genetics. Barcodes.
BS: Right. I’ll let Tony handle this.
TZ: Right, so how do we barcode the neurons? Well, turns out that if you want to deliver a gene to a neuron, a gene that that neuron doesn’t normally express, what you can do is make a virus that encodes whatever it is you want to express.
BS: It’s a pretty common technique, and these viruses are not the kind that can get you or I sick. All the machinery that normally helps a virus wreak havoc on a cell has been taken out, and all that remains is a hallow shell.
TZ: If you like gutted viruses and the space left over, the space created by gutting them, we use for inserting the cargo of interest that we would like to deliver to the neurons.
BS: Each of Tony’s virus particles carries a unique barcode sequence made up of 30 RNA letters or nucleotides. When the virus is injected in a brain region of interest, unique barcodes are taken up individually by neurons in that region.
AA: So now you’ve got a bunch of neurons individually barcoded. That’s obviously not the end of it though. If I were to go back to the airline metaphor, that’s just bunch of marked airplanes sitting at their terminals. What happens next?
BS: Next, like with a lot of science, you wait.
TZ: 48 hours. This is a fast acting virus, so in 48 hours, the virus can infect the neuron, can cause this protein to be expressed, cause the barcode to be expressed, and it also even drives the barcodes out to the synapses.
AA: Synapses are where the signal carrying cables of neurons meet.
BS: Yeah, and those cables are what neuroscientists call axons. Now, to find out where those barcodes went.
AA: That is, what other neurons are at far end of the axons sent out by the barcoded neuron.
BS: Right. To do that, Tony and his team, just like all mappers of the mouse brain, have to sacrifice the test animal, and in this case, find out where in the brain all those barcodes were carried by the virus, the place where each neuron’s axon formed a synapse with another neuron. But instead of looking for a visual trace, as with optical methods that can only trace one or two neurons per animal per experiment, Tony’s method uses RNA sequencing to find the final synaptic destinations of the many thousands of barcodes that infected neurons in the source region, that original brain area of interest.
TZ: And that way, we can get more information in a single experiment than anyone has ever had before. We can get the [all-to-all 00:19:17] conic activity of about 50,000 neurons from a single specimen, and we can get that at single neuron resolution.
AA: Wow, and this is all in about 48 hours and using one mouse and not thousands? Yeah, I can see how this is a game changer.
BS: Still, there’s a lot of questions left to ask. One big one being, “So what can we gain from this?”
TZ: And the answer is we don’t know what we’re gonna know, so if you asked 15 years ago when people were sequencing the human genome, “What good is the human genome gonna be?” There are a lot of possible answers. In the end, the day that the human genome was released, I would say, there was no real headline. The headline that I read was, “Human Genome Has About 20,000 Genes,” but you know what, my life was no different with the knowledge that it had 20,000 genes, not 5,000, and not a 100,000.
So the day the human genome was released, I don’t think we knew that much. That said, genomics has transformed how we do biology. It has provided a solid foundation. It’s changed the way that everything in modern biology is done. I suspect that it’ll be the same when we have the Connectome. So in the most optimistic scenario, the day that we finish the mouse Connectome, we’ll slap our foreheads and say, “Ah! Now it’s obvious. Now we see that that’s how the brain works.”
And sure, I sure hope that’s the case, but I don’t think so. I think instead, what it will serve to do is constrain our hypotheses about how the brain works. We’ll know that no, it can’t possibly be this way because the circuitry is just not available for that, and it’ll guide us toward thinking of other classes of hypotheses.
So in five years, when we have the Connectome of a mouse, we’ll start a set of experiments by sitting down at a computer and just sort of thinking through some of the circuitry that might be involved in whatever behavior we’re interested in. And immediately, we’ll rule out 99 out of our hundred choices or maybe 98, and maybe we’ll be left with 2 or 3 or 4, and those will be the ones that we’ll spend our time actually studying with other sorts of tools.
So that’s how I think it’s going to impact what we do.
AA: What I’ve gleaned from all this is that even with a Connectome, even with a high-resolution atlas of an entire brain, we still don’t have enough to spark a revolution in drug discovery. It’s a revolution of another kind, a necessary change in how neuroscience will be done moving forward.
BS: That’s right. It may be that neuroscience has to evolve first to start asking new questions, and it will be the answer to those questions, questions we don’t even know yet, that will set the stage for a neuropsychiatric revolution. It’s not gonna happen overnight, but I’m personally convinced that Tony is at the starting point of this new journey.
AA: And as for our friends, Ray and Rob, what do they think of all this?
RH: And I think that may be the reality, that we just have to wait for people like him to do their work and come up with the beautifully elegant maps that the rest of us can then use to cherry-pick targets, where I can say there’s a simple-minded drug discover, “This is a terrific hypothesis, let’s try and discover a drug that works here.” But you know, you can’t do it until the work is completed.
RM: So I think the kind of foundational Connectome mapping that people are doing, I think we have to do it. I am a passionate believer in that none of us is smart enough to know where the big breakthroughs are come from, and the best use of money is to support the best basic science on issues relevant to neuropsychiatric disorders because that’s where, if you look at the history of biomedical research, that’s where major breakthroughs come from.
Blah blah blah. I’ll shut up finally.
BS: So, I could talk to Rob for the rest of the day, but we usually try to keep these episodes short and sweet.
AA: But you know, maybe you guys want to hear more from us. Maybe you’d like longer episodes, and we want to know that, so we’d love for you guys to review us on iTunes and let us know what you guys are liking, what you’d like to hear more of. And this is a really great time to do that because-
BS: It’s the last episode of the season. We’re gonna take two month siesta?
AA: Yeah, we’ll be back on March 15, 2017.
BS: 2017.
AA: It’ll be next year already.
BS: And our New Year’s Resolution will be to improve this podcast, so hop over to iTunes, hop over to Lab Dish and please tell us what you think.
AA: Yeah, please. We really, really would love to hear from you.
Extras for Episode 7
The atlas expanded
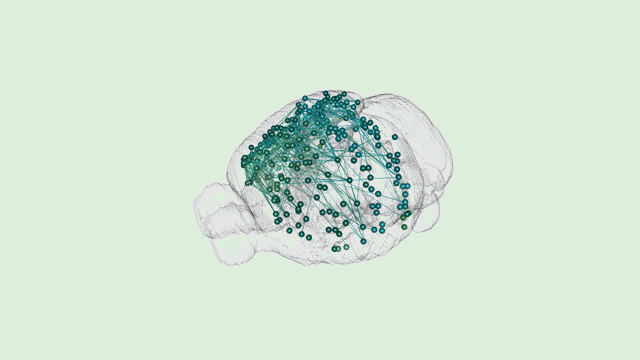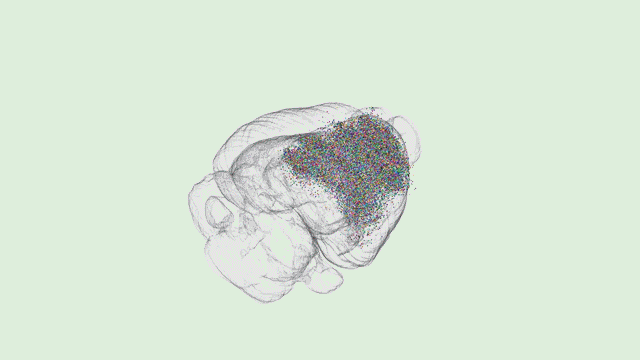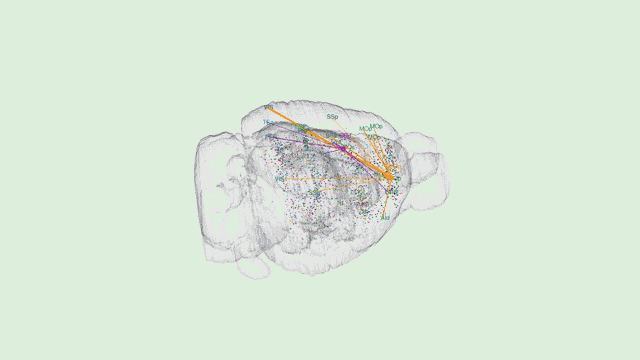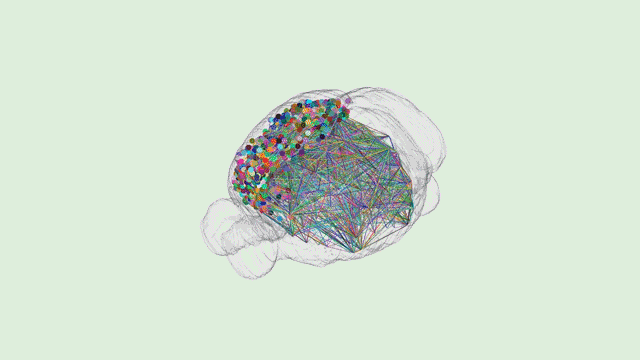
Dr. Tony Zador’s MAPseq method could very well be revolutionary—capable of generating dozens of “maps” of different parts of the brain at a single-neuron resolution. However, Tony will be the first to tell you that his work is only the beginning.
“In general, when new techniques come along, there’s a while where the community sort of figures out what the relative strengths and weaknesses are and then ends up using that new technique in some collection of cases,” Zador explains.
“Certainly there are some weaknesses of our approach,” he added. “The biggest weakness right now is spatial resolution. The advantage of traditional microscopy is that you can actually see the neuron and its fine dendritic processes and the precise trajectory of each one of the axons.”
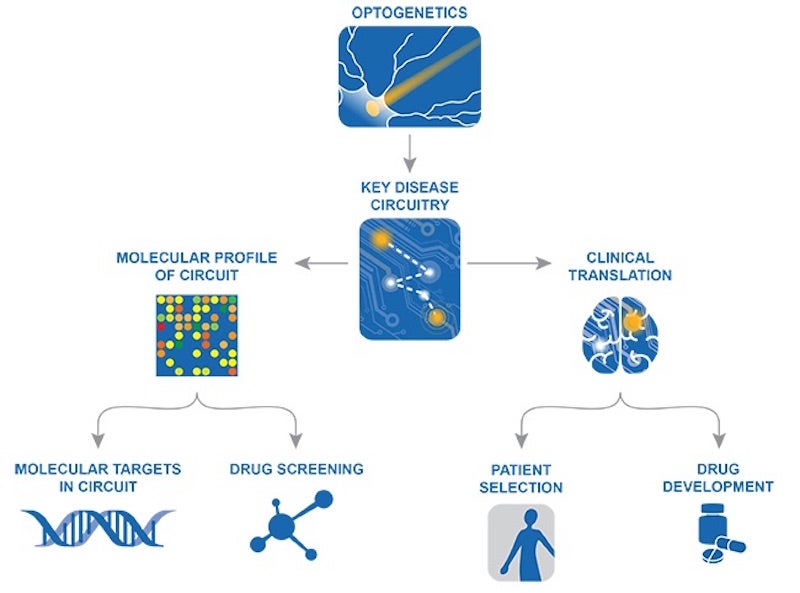
Seeing individual neurons in action—understanding their trajectory, purpose, and even class—is work that falls more into Dr. Robert Malenka’s realm.
Stanford professor and co-founder of Circuit Therapeutics, Malenka strongly believes in the value of optogenetic drug discovery. It’s an approach that harnesses modern technologies to identify and address broken circuitry on a molecular level—something that can only be achieved through a deep understanding of how each class of neuron should be functioning within a circuit. MAPseq and other connectome projects could help Rob and his colleagues find these troublesome targets.
One other obstacle
In addition to troubles with the pharmaceutical industry itself, Dr. Raymond Hill—former Executive Director, Licensing and External Research, Europe, for the pharmaceutical giant Merck, suggests there could be another factor holding back neuropsychiatric drug discovery.
Academia, he explains in our interview, may have become so overspecialized that drug discoverers are getting “tunnel vision.”
“I think if you look back to the great empirical scientists of the past—people like Paul Janssen—they were clinicians, they were biologists, they were organic chemists. They seemed to have their finger in many pies,” Hill says. “Although they may not have been true world-class experts in everything they knew about, they were capable of putting all the pieces together and coming up with something that turned out to be a useful drug.”
“Whereas these days we all know more and more about less and less!”

(Credit: Imperial College London, Stanford Medicine, Max Gerber)
According to Hill, even in an age where a bottomless pool of information is quite literally at the tips of our fingers, it’s easy for scientists to forget there are other sources of data and perspective outside of their own field.
“If I don’t really know what it is I’m looking for and I’m groping around in the dark, then a chat with the chap in the next lab over a cup of coffee may actually give me that inspiration at least to know what to search for on the internet.”
It’s a problem that many scientists have trouble surmounting. However, here at Cold Spring Harbor Laboratory, collaboration is not only encouraged, but celebrated! With its dozens of annual meetings and courses, symposia, and even the first preprint server for the biological sciences, CSHL endeavors to have a quickening impact on promising ideas and projects across the life sciences.
Sharing the bounty
And what about MAPseq? In the spirit of sharing knowledge, Zador’s lab will continue to share all their results on BioRxiv. What’s more, they hope to provide MAPseq as a service to other labs in the near future.
“In fact, we’re setting up a facility here at Cold Spring Harbor to provide MAPseq as a service to labs that are interested in using it to study whatever connections they’re interested in,” Zador said. “Currently we have a collection of collaborators that we’re working who are applying it to questions like the development of the visual system, the local architecture of the visual system, the architecture of some neural modulatory centers and so forth.”
Want to hear more from Tony? Check out his recent public lecture below!
Written by: Brian Stallard, Content Developer/Communicator | publicaffairs@cshl.edu | 516-367-8455