Selective targeting of endothelial progenitor cells blocks metastatic progression
Cold Spring Harbor, NY — Cancer patients usually ask what can be done after a primary tumor has already spread, or metastasized, to other organs. In many cases, they learn, little can be done. Hence the importance of a discovery by scientists at Cold Spring Harbor Laboratory (CSHL) of a type of cell that regulates the transformation of small, dormant lung metastases into large, aggressive metastases—the kind that kill cancer patients.

The cells that promote the metastatic transformation are called endothelial progenitor cells, or EPCs, and are found in the bone marrow. The CSHL research team reports in the January 11 issue of Science that EPCs regulate an “angiogenic switch”—a key mechanism that causes formation of blood vessels in tumors and triggers tumor growth.
“A majority of malignant primary tumors have already spread to other organs by the time they are clinically diagnosed,” noted Vivek Mittal, Ph.D., head of the CSHL research team and corresponding author of the Science paper. “Current efforts are focused on preventing metastatic spread, yet, paradoxically, insights have been lacking on how dormant metastatic lesions, after they have colonized distant organs, grow into large, lethal lesions.”
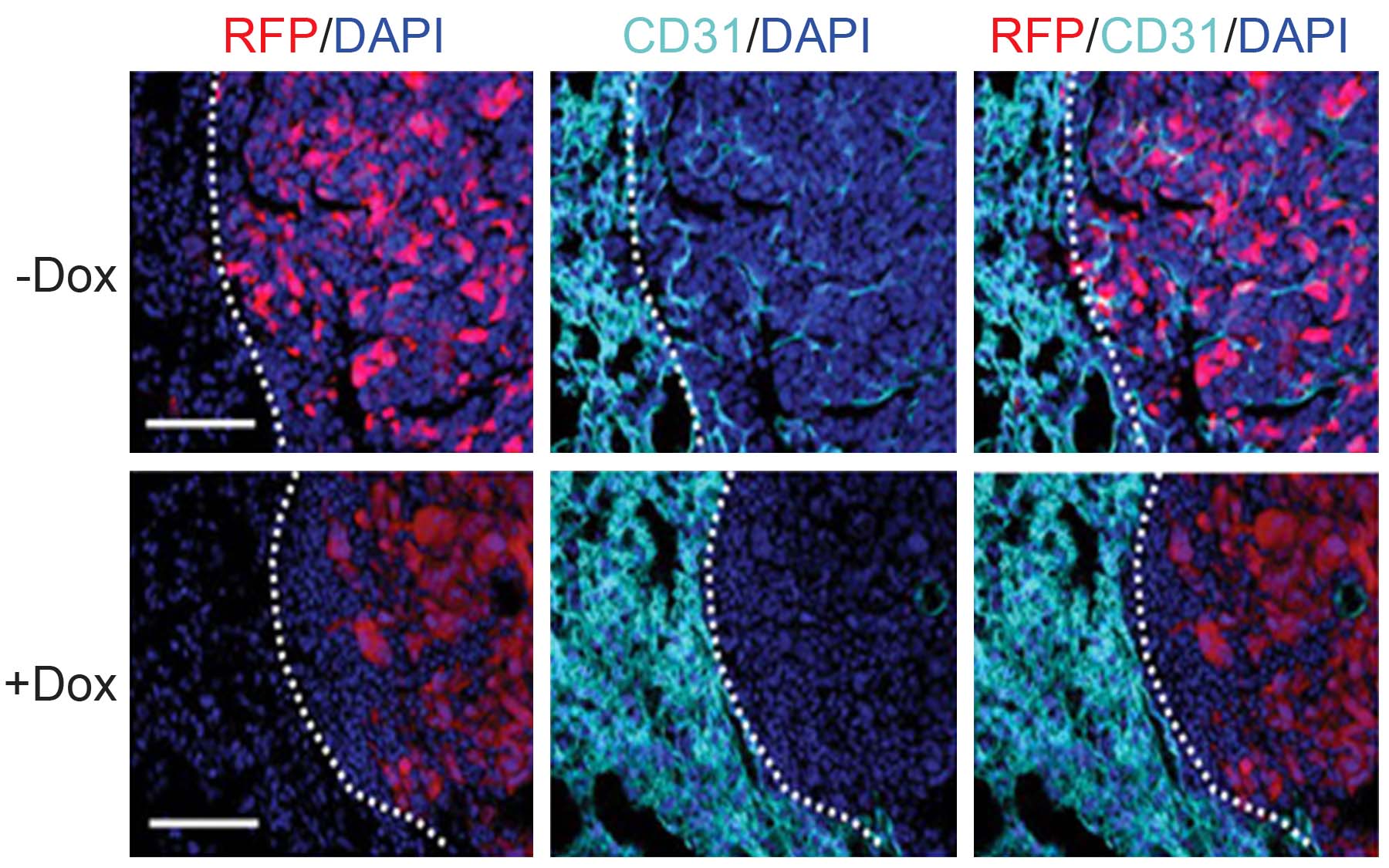
“Our study has focused on cells from primary tumors in mice that have spread and established micrometastases in secondary organs such as the lung,” said Dingcheng Gao, Ph.D., a CSHL postdoctoral fellow and lead author of the Science paper. “We’ve dissected the heart of the angiogenic switch and demonstrated that micrometastases recruit EPCs from the bone marrow. These EPCs, in turn, regulate the angiogenic switch that activates blood-vessel growth and transforms these dormant lesions into life-threatening macrometastases.”
Drs. Mittal, Gao and colleagues at CSHL showed in experimental mice that levels of a protein called Id-1 increase dramatically in EPCs when tumors are present. By using a technique called RNA interference, or RNAi, to block the expression of Id-1 in living animals, the team was able to prevent mobilization of EPCs to the site of metastasis, and thereby inhibit the angiogenic switch. This, in turn, interrupted the process in mice by which micrometastases are converted into lethal macrometastases. Notably, increased survival was noted in the tumor-bearing animals that were treated with this method. The next step is to perform a similar study in humans.
“This study has raised the prospect of a novel therapeutic target, and suggests that selective targeting of EPCs, perhaps in combination with chemotherapy, may prove to be a clinically feasible approach in the treatment of people diagnosed with cancer that has metastasized to the lungs,” Dr. Mittal said.
“Past experiences have highlighted the challenges associated with therapies that target genetically mutant cancer cells. For instance, we know that cancer cells develop resistance to chemotherapeutic agents. We feel that approaches based on targeting the genetically stable components of the tumor microenvironment, such as the EPCs, need to be further explored for effective treatment of cancer.”
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455
Citation
“Endothelial Progenitor Cells Control the Angiogenic Switch in Mouse Lung Metastasis” appears in Science on January 11. The compete citation is as follows: Dingcheng Gao, Daniel J. Nolan, Albert S. Mellick, Kathryn Bambino, Kevin McDonnell and Vivek Mittal. The paper is available online at: http://www.sciencemag.org/cgi/content/abstract/319/5860/195