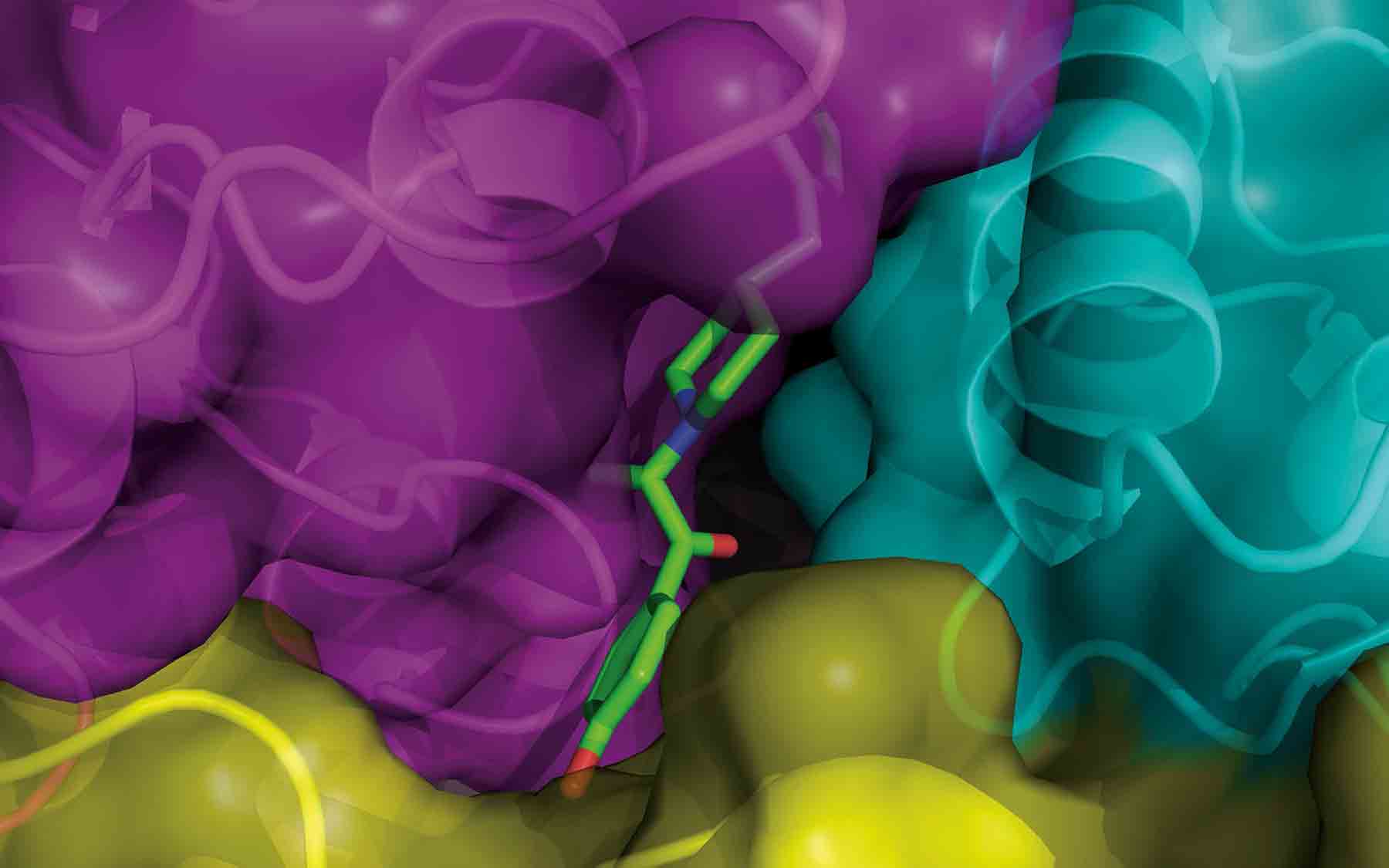
This computer-generated 3-D image, from Erkan Karakas, Ph.D., a postdoctoral researcher in the lab of CSHL Associate Professor Hiro Furukawa, reveals in exquisite detail the shape of a previously elusive binding pocket for a drug that may help treat neurodegenerative diseases including Alzheimer’s and Parkinson’s, as well as brain damage from stroke.
The neuroprotective drug is called ifenprodil. It’s the green “stick” you can half-glimpse through a tiny opening in the structure in which it is embedded (loops and ribbons represent amino acids forming the structure). The binding pocket is like a keyhole, where only molecules of a very particular shape and electrical charge can fit. This view shows how ifenprodil binds within one pocket in one portion of a type of NMDA receptor. Found throughout the human brain, NMDA receptors are tiny pores whose opening and closing helps regulate signals—excitatory nerve impulses—between cells carried by the neurotransmitter glutamate.
This rendering of the amino terminal domain (ATD) of an NMDA receptor has significance for drug developers. “When you can see where, how, and how well a candidate drug fits in a binding pocket, you can begin to tweak it to make sure it binds only there and not at other receptors in the body, where it can cause harmful side effects,” Karakas explains. Two years of work went into this solution of the structure, using a method called x-ray crystallography.
The team deduced that movement of the lower (yellow) of the two linked protein assemblies on the ATD’s right side (cyan and yellow, comprising the GluN2B subunit) enables ifenprodil to enter and exit the tiny pocket. One experiment in the series proved that binding at this “allosteric” site in the ATD shuts down the larger NMDA receptor—important because NMDA overactivity is linked with neurodegeneration. The way is now open for development of an ifenprodil-like drug that binds even more exclusively at this site, minimizing side effects.
Written by: Peter Tarr, Senior Science Writer | publicaffairs@cshl.edu | 516-367-8455