Cold Spring Harbor, NY — Malignant melanoma is an aggressive, deadly cancer that does not respond to conventional chemotherapy. Other aggressive, chemoresistant cancers—and approximately half of all cancers—are characterized by mutations in the p53 tumor suppressor gene. Malignant melanomas, however, do not typically display mutations in the p53 gene.
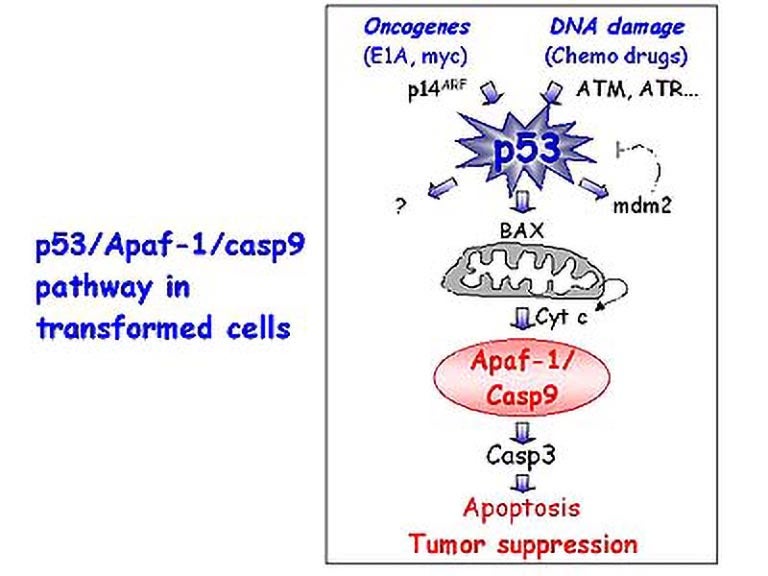
To explore alternative explanations for the origins and properties of malignant melanoma, and to identify potential targets and strategies for therapy, Scott Lowe and his colleagues at Cold Spring Harbor Laboratory have examined the status of other genes known to function downstream of p53 in a pathway leading to “apoptosis” or “programmed cell death.” When intact, this pathway rids the body of abnormal, pre-cancerous cells by triggering a cellular self-destruct mechanism. When this pathway is disrupted (by the loss of p53 function, for example), pre-cancerous cells survive and proliferate resulting in cancer (see figure).
In a study to be published tomorrow in Nature, Lowe and his colleagues report that malignant melanomas often lose a key trigger of programmed cell death – a protein called Apaf-1 (apoptosis activation factor-1). The researchers also discovered that the loss of Apaf-1 in melanoma cells is associated with resistance to the chemotherapy drug adriamycin. Most significantly, the study shows that restoring Apaf-1 in melanoma cells rescues the ability of these tumor cells to kill themselves in response to adriamycin.
“The loss of Apaf-1 in malignant melanoma is a prime explanation for both the extreme chemoresistance and the apparent lack of p53 defects in such cancers,” says Lowe.
The study is significant because it demonstrates that accurate diagnosis and treatment of malignant melanoma, and perhaps other cancers, should include an assessment of the status of Apaf-1.
Maria Soengas of Cold Spring Harbor Laboratory was the principal author of the study. The principal collaborators in the study were William L. Gerald and Carlos Cordon-Cardo of Memorial Sloan-Kettering Cancer Center.
An intriguing aspect of the study relates to the mechanisms that cause the loss of Apaf-1 in melanomas. The researchers were not surprised to find that one of the two copies of the Apaf-1 gene was deleted in many of the melanomas they examined (a well-known phenomenon called “loss of heterozygosity”). However, the other Apaf-1 gene copy in these cells was present and apparently normal in terms of its DNA sequence. Interestingly, Lowe and his colleagues found that complete loss of Apaf-1 was due to “transcriptional silencing” of the remaining, normal copy of the Apaf-1 gene in melanomas. Transcriptional silencing, or the suppression of gene expression due to higher order chromatin structure, is emerging as a principle mechanism whereby Apaf-1 and other tumor suppressor genes can be inactivated, resulting in cancer when other copies of such genes are lost, mutated, or similarly silenced.
Scott Lowe is a Professor at Cold Spring Harbor Laboratory and Deputy Director of the Cold Spring Harbor Laboratory Cancer Center. Other scientists from Memorial Sloan-Kettering Cancer Center (Paolo Capodieci, David Polsky, Jaume Mora), Johns Hopkins University and Oncology Center (Manel Esteller, James G. Herman), and Cold Spring Harbor Laboratory (Ximena Opitz-Araya, W. Richard McCombie, Yuri A. Lazebnik) contributed to the study.
Pathway leading to apoptosis or programmed cell death – DNA damage (such as that caused by certain chemotherapeutic drugs) and other signals stimulate p53. As a result, Apaf-1 is activated, triggering apoptosis. When this pathway is disrupted, as by loss of p53 or Apaf-1 function, pre-cancerous cells survive and proliferate, and eventually form a tumor. The pathway also functions naturally to generate shapes and control the number of cells in various tissues.
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455