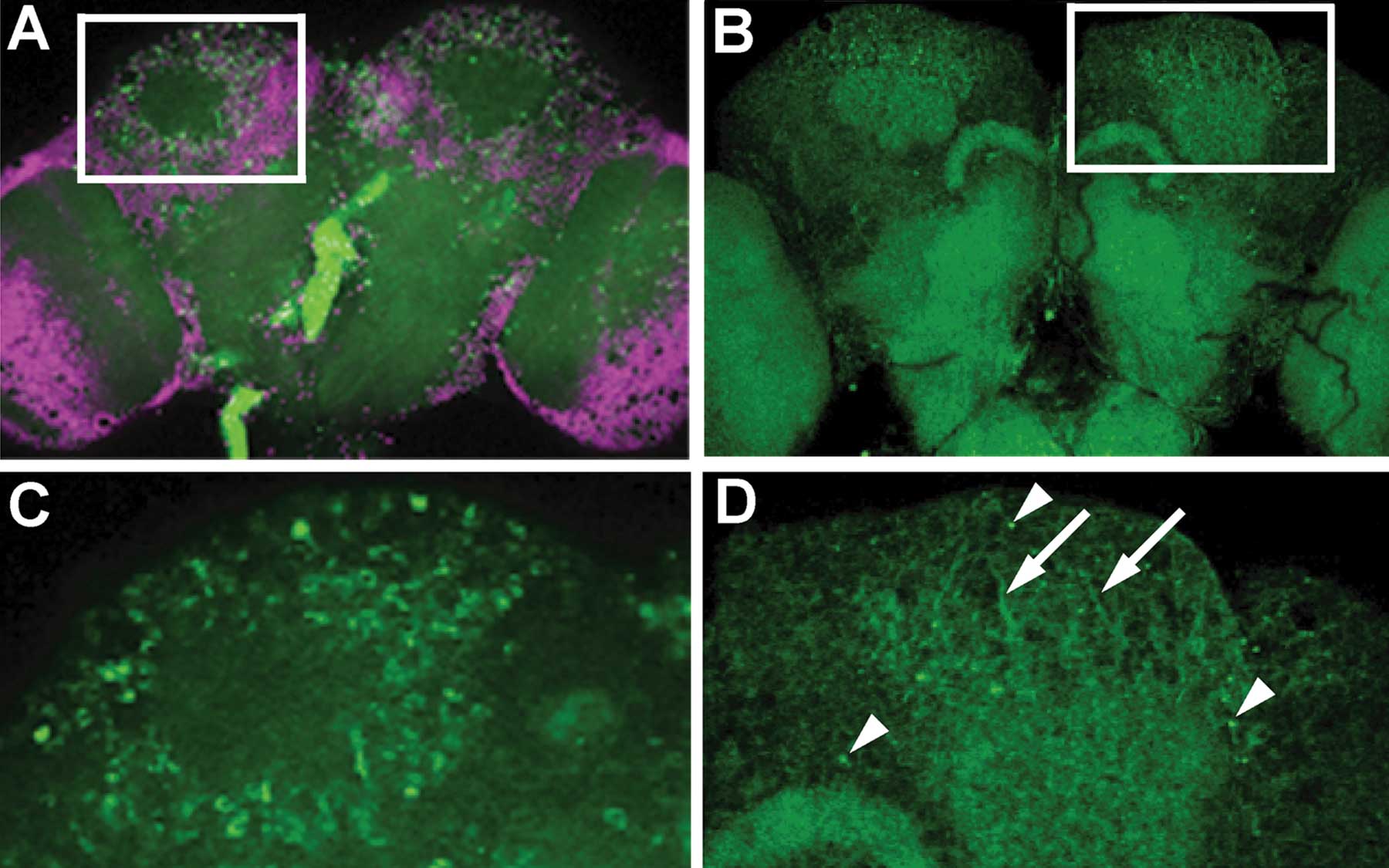
One of the 13 core papers identified within the area of “Early Alzheimer’s Disease” (disease appearing in people in their 40s and 50s) is a 2008 study published by CSHL’s Dr. Yi Zhong. In the paper, Dr. Zhong and his team focused on the aggregation of the amyloidβ42 peptide (Aβ42) in the brain, a pathological hallmark of Alzheimer’s and a good target for therapeutic intervention.
Some humans carry mutations that increase the peptide’s tendency to aggregate. Studies on cells grown in the lab suggested that such increased aggregation might result in higher neurotoxicity and worse disease pathology. But other evidence suggested that this “relationship between Aβ42 aggregation and neurotoxicity and pathology might not be such a straight line,” explains Zhong.
Aβ42 can form a whole range of misfolded structures in the brain, so Zhong hypothesized that these many forms or Aβ42 “species” might aggregate differently and trigger different toxic effects and disease phenotypes. His team’s experiments on fruit flies engineered to produce the normal human Aβ42 or either of two mutant Aβ42 peptides—one that tends to aggregate more than normal, and the other, less than normal—confirmed Zhong’s idea.
While the peptides that aggregated more caused more severe locomotive dysfunction and premature death, the mutant peptide that aggregated less caused earlier onset of memory defects. The three different peptide versions also caused different levels of neuron loss and degeneration.
“Our discovery that Aβ42’s complex toxicities are associated with different aggregation propensities suggests that trying to treat Alzheimer’s with aggregation inhibiting drugs may simply modify Aβ42’s pathogenic effects without halting or reversing it,” explains Zhong.
The differences in pathology, the paper showed, stem from differences in how the peptides are distributed intracellularly within the brain’s neurons. In other words, the potentially reversible early symptoms of Alzhemier’s could be mediated by the intracellular accumulation and aggregation of Aβ42, which suggests that targeting this process might be a better treatment strategy for early Alzheimer’s disease. In the meantime, Zhong’s team has continued to explore the mechanism of Aβ42 toxicity using the fruit fly as a model system to identify the immediate downstream targets of Aβ42 aggregates, information they hope to publish soon.