The target, PRC2, is a lysine methyltransferase-containing protein complex with components that can be readily targeted with small molecule inhibitors
Cold Spring Harbor, NY — Scientists at Cold Spring Harbor Laboratory have identified a candidate drug target for treating acute myeloid leukemia (AML), a white blood cell cancer that proliferates out of control in the bone marrow. The team, led by Assistant Professor Chris Vakoc, M.D., Ph.D., shows that blocking a protein called PRC2 halts this uncontrolled proliferation in the bone marrow of mice with AML.
PRC2, short for Polycomb Repression Complex 2, is a chromatin regulator—a type of protein that alters the way genes are expressed. PRC2 works by chemically modifying histones, which are proteins that associate intimately with DNA to regulate how it functions in the cell. The team has found that blocking PRC2 leads to the reactivation of a powerful anti-tumor pathway that can protect against AML. “Our results emphasize the idea that targeting chromatin regulators is an effective means of reawakening growth-halting mechanisms that lie dormant in most cancer cells,” says Vakoc. The study appears online in Oncogene on April 2.
AML is caused by changes not only in the genome but also the epigenome, which can be likened to a chemical instruction manual that contains reversible or rewritable instructions on how genes are expressed in cells. “If you think of the genome as something that’s hardwired, then the epigenome is something that can be rewired,” explains Vakoc. A growing body of evidence suggests that AML can arise from dysregulation of the epigenetic machinery that controls chromatin.
Using an RNA interference (RNAi) screen in which small hairpin-shaped RNA were used to systematically switch off every known chromatin regulator, Vakoc’s team, in collaboration with the lab of CSHL Adjunct Professor Scott Lowe, Ph.D., looked for signs of inhibition of the growth of leukemic cells but not normal cells. The team’s previous use of this approach identified a potent target called Brd4, a drug for which is currently in pharmaceutical development. Much of the technology for RNAi screening has been pioneered at CSHL by the laboratory of Gregory Hannon, Ph.D.
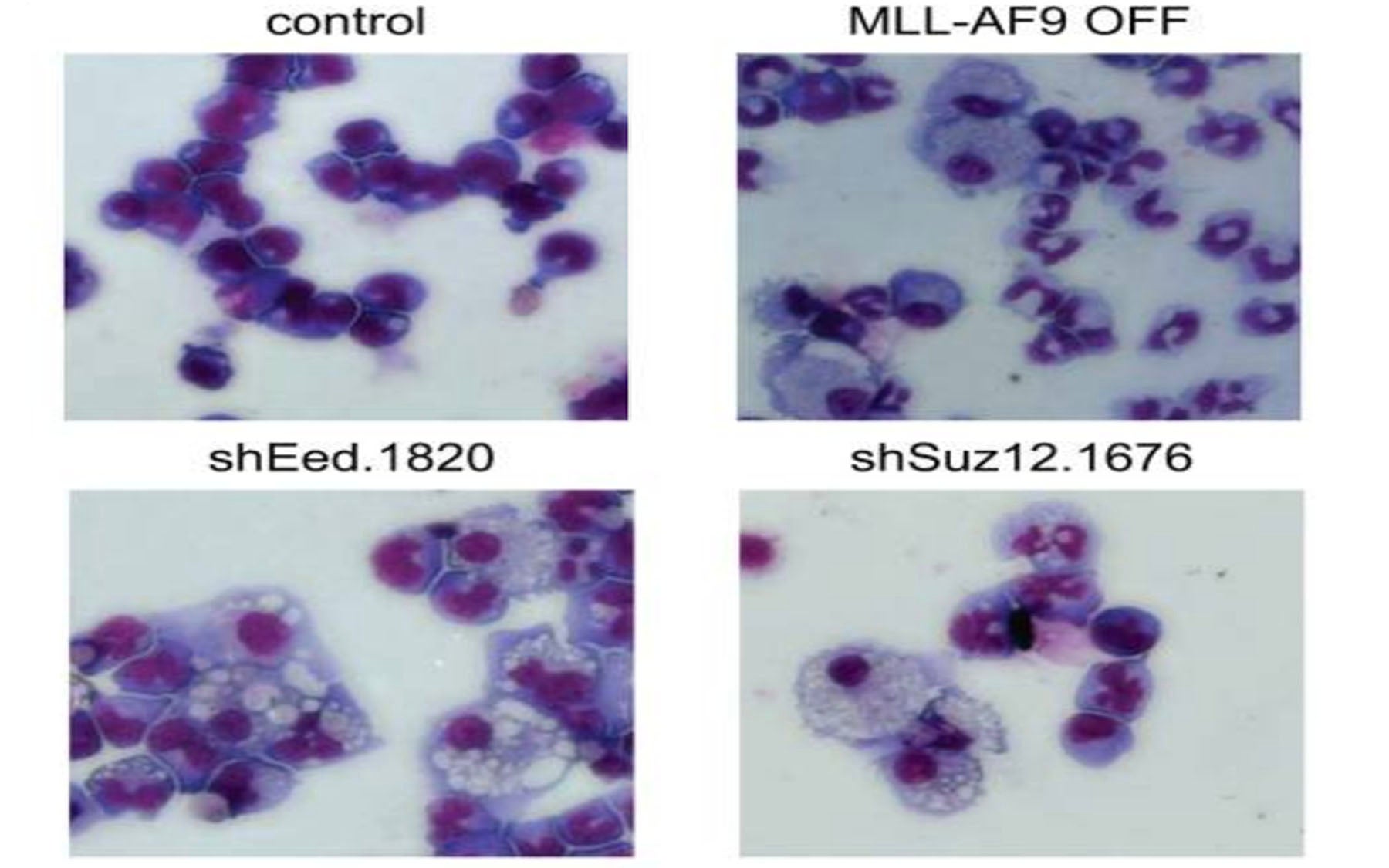
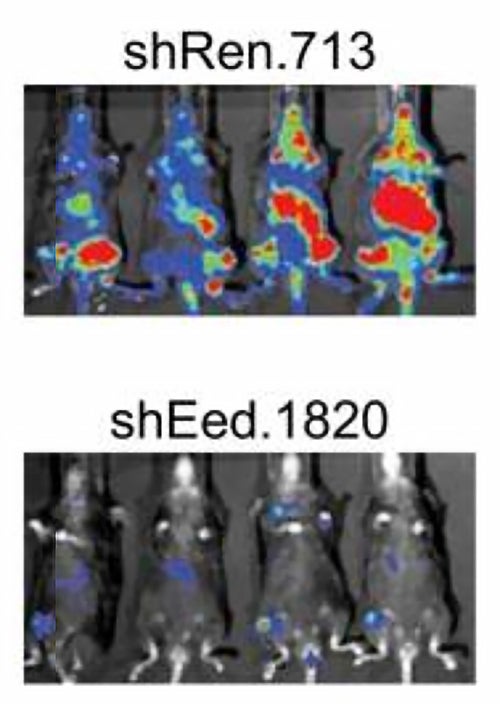
“In our new screen, PRC2 was one of the few chromatin regulators that was needed by cancer cells, but dispensable for normal blood cells,” says team member Eric Wang. Blocking PRC2 led to growth arrest of leukemic cells and their differentiation—the loss of ability to self-renew.
Although the screen also turned up other potential drug targets when performed in vitro, i.e., in cells grown in the lab, PRC2 was one of the few targets whose inhibition stopped the growth of leukemic cells in the bone marrows of mice with an aggressive form of AML. “These advanced mouse models, pioneered by former CSHL post-doctoral fellow Johannes Zuber while he was in Scott Lowe’s laboratory, have given us the ability to discriminate between unsuccessful targets that only work in plastic dishes and those that are effective inside animals,” says Vakoc.
The lack of such preclinical evaluation and validation is one reason why so many drug trials fail. The use of cancer mouse models as preclinical test-beds, as described in the current study, is a cornerstone of CSHL’s Cancer Therapeutics Initiative, which guides drug development by prioritizing drug targets that are identified by RNAi screens.
“We found that targeting the PRC2 complex allows reactivation of Ink4/Arf, genes that control a powerful tumor suppressor pathway that becomes silenced in AML. This provides a clear rationale for a chromatin regulator as a drug target in this disease,” says graduate student Junwei Shi, who performed these experiments.
Both cancer-causing oncogenes as well as tumor suppressor genes, which are frequently missing or inactivated in AML, are often challenging to target with small molecule inhibitors. “Epigenetic regulators such as PRC2, on the other hand, are considered druggable and therefore are good surrogate targets,” explains Vakoc. “Targeting Brd4, for example, is a great way to switch off the Myc oncogene. And as this study shows, targeting PRC2 is a great way of switching on the Ink4/Arf tumor suppressors.”
The druggability of PRC2 lies in its identity as a lysine methyltransferase, a type of protein with deep greasy pockets in its structure that are accessible to small molecule drugs that can block its activity. Lysine methyltransferase inhibitors are already being developed as candidate anticancer drugs by many pharmaceutical companies.
Written by: Hema Bashyam, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
The work was funded by support from the Don Monti Memorial Foundation, the Laurie Strauss Leukemia Foundation, the Sass Foundation, the Edward P. Evans Foundation and the FM Kirby Foundation.
Citation
“The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;NrasG12D acute myeloid leukemia,” appears online on April 2. The full citation is: J Shi, E Wang, J Zuber, A Rappaport, M Taylor, C Johns, SW Lowe and CR Vakoc. The paper can be downloaded at http://www.nature.com/onc/index.html using the doi: 10.1038/onc.2012.110.
Principal Investigator

Chris Vakoc
Professor
Alan and Edith Seligson Professor of Cancer Research
Cancer Center Deputy Director of Research
M.D., Ph.D., University of Pennsylvania, 2007