Autism can sometimes be inherited, but exactly how is complicated. CSHL Professor Michael Wigler defied the field’s common wisdom in the mid-2000s to figure out some of the genetics underlying autism. It turns out that dogs can help explain Wigler’s approach. In a video call conducted March 20, 2020, Wigler mused about how remarkable it is that dogs—a single species—come in so many sizes:
“Let’s think about the size of dogs. Okay there are really tiny dogs like four pounds and you got Great Danes that can be 150 pounds. The dog has figured out—the species has figured out—how to modulate its size. Totally not trivial. Y’know that all the physiology kind of works over that huge range.”
Dogs have a genetic toolkit to allow for a wide range of sizes. Many genes make up that toolkit, and versions of those genes need to work together to produce dogs of varying sizes and shapes.
Wigler thinks that the human brain has a similar toolkit, allowing us to adapt over only a few generations. When the environment remains stable for a long time and people are doing fine, there would be little need for brain variation. The brain is already well adapted for the situation. But if the environment shifts, the population would benefit from having a broader range of brains, on the chance that a new set of features would work better in the new situation: “You would want that in times of great change to vary the development scheme so you get diversity.” Autism represents what happens when brain systems that should integrate well with one another don’t work together optimally, perhaps a genetic adaptation trick gone awry.
As research on autism progressed in the early 2000s, it became clear that at least some cases of autism had a genetic basis, but the mechanism for inheritance was not the same as eye color or height. Wigler was not deterred by this complexity. He is a molecular biologist with an undergraduate degree in mathematics who spent his early career studying the genes that can either cause or suppress cancers. Cancer genetics is very complicated. Wigler saw that cancer genetics might help him approach autism genetics.
Michael Wigler joins Far from the Tree book author Andrew Solomon to discuss autism research with parents of children who are different. The exchange was filmed at an event at the Cinema Arts Center January 9, 2019 after a showing of the documentary film Far from the Tree.
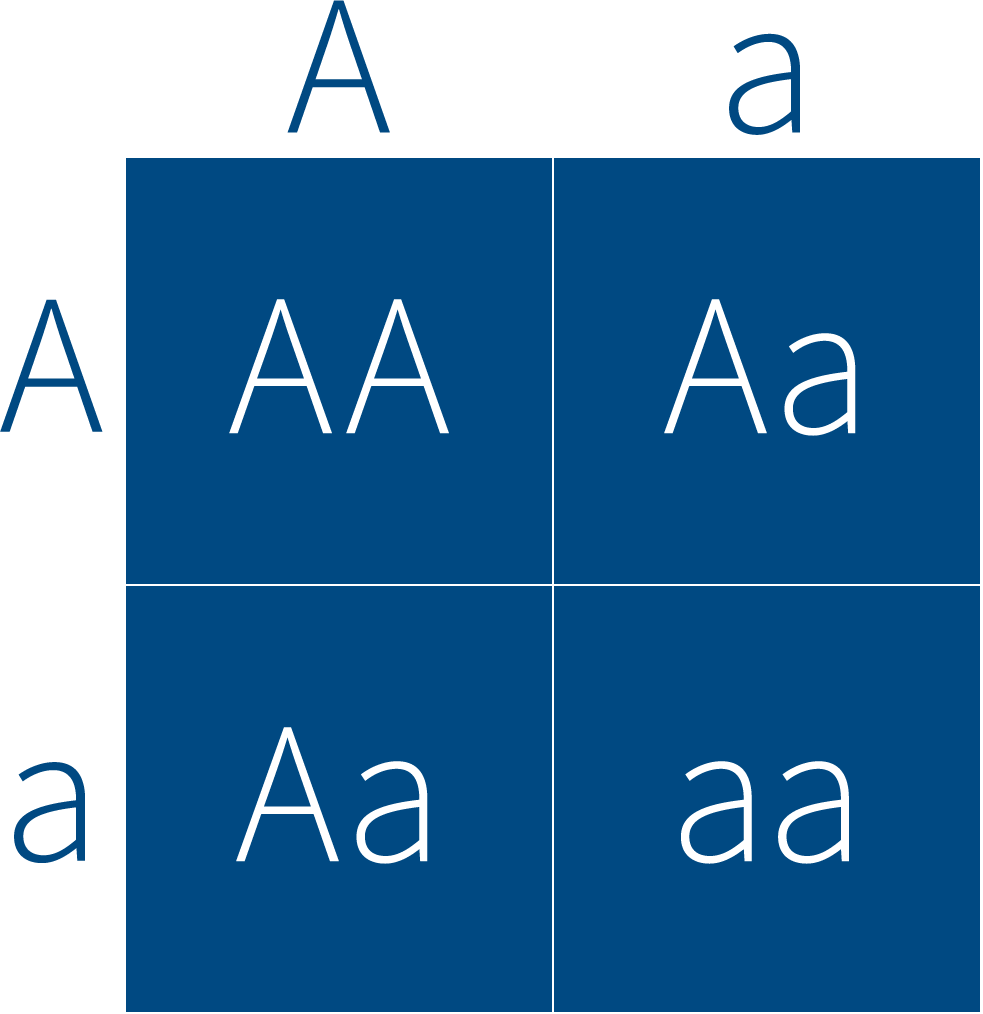
Traits like eye color or height or even disease are passed from parents to children through versions of genes—alleles—that can behave in one of two ways. Recessive alleles must be inherited from both mother and father to cause the trait—say a disease—to appear in a child. One-quarter of the children have the disease. Dominant alleles require only one parent to pass on their allele. In that case 50% of the children have the disease. For parents carrying a dominant allele, the risk to any future child—the sibling risk—is also 50%.
But that does not explain all human genetic variation. In 2004, Wigler discovered that some genes could be duplicated or deleted when being transmitted from parent to child—a phenomenon he dubbed copy number variation (CNV). This could happen in the creation of sperm or eggs, resulting in a child inheriting an abnormal number of copies of a single gene from one parent, who had no such copy number variation in his or her genetic code.
In the mid-2000s, researchers calculated the sibling risk for autism to be something like 10%, but that left a lot of questions about the way autism was inherited. Many thought it meant that autism must be caused by multiple recessive alleles. If enough bad alleles were inherited across several genes, a child would have autism.
“That was a theory I never liked,” Wigler said, “because it wasn’t provable and it didn’t match with a broader view of the genetic landscape. The genetic landscape contained high sibling risk. But just looking at the overall sibling risk told you nothing. You could get a 10% sibling risk if in some families the sibling risk was close to zero, and in a small number of families was close to 50%.”
In other words, an overall sibling risk of 10% might be calculated from a population of autism patients, most of which have low risk and only a few with very high risk in a small number of genes. In 2007 Wigler’s lab found an explanation, reporting that spontaneous germline mutations contribute to autism. In these cases, parents had one set of genes, but the genes in their sperm or egg cells had brand new mutations, often duplications or deletions. Wigler noticed, “There was an extremely clear observation: in fact if you already have two kids with autism the chance of the third one, if it’s a boy, having autism was 50%. And it was just lying around in the data, in plain sight.”
But not all autistic children are born with first-generation mutations. Some families carry an elevated genetic risk for autism over several generations. What remains a mystery is why some people can carry the gene without being autistic themselves. “There are two ways a parent can be a carrier,” enumerates Wigler. “One is if the parent is female, because females are somewhat resistant. And the other is if there were some unknown factors, some factors that we don’t know that we’re still searching for, that could render somebody apparently normal but carry what would be devastating in another background. And that’s sort of the Holy Grail right now for autism. Both to understand the female resistance, which is a very hard problem, and to find genes that could render even males resistant.”
In 2007, Wigler published his “unified theory” for autism (pdf) including both brand new “de novo” mutations and transmission from parent to child. Most cases of autism were caused by new mutations in the parents’ sperm or eggs. Sons who inherit the mutation are far more likely to express autistic characteristics than daughters, who for reasons still not understood are resistant to the effects of that gene. Females can pass on the mutation with a probability of 50% to their offspring. New mutations and mutations passed through resistant, often female, carriers explain most cases of autism. The theory explains why autistic children are more often born to older parents, since older parents tend to display more germline mutations.
In the years since, Wigler and his colleagues showed that many genes work in concert to build our brains. Just as hearts and lungs adjust to accommodate tall dogs and short dogs, wide dogs and thin dogs, our brains need to tune many systems simultaneously to function optimally. Autism shows what happens when at least some brain pathways are not optimized to work with other systems.
Written by: Eliene Augenbraun, Creative Director | publicaffairs@cshl.edu | 516-367-8455
Principal Investigator

Michael Wigler
Professor
Russell and Janet Doubleday Professor of Cancer Research
Cancer Center Member
Ph.D., Columbia University, 1978
