Precisely mapped allosteric binding site will facilitate design of drugs with minimal side effects
Cold Spring Harbor, NY — Structural biologists at Cold Spring Harbor Laboratory (CSHL) have obtained a precise molecular map of the binding site for an allosteric inhibitor in a subtype of the NMDA (N-methyl-D-aspartate) receptor, which is commonly expressed in brain cells.
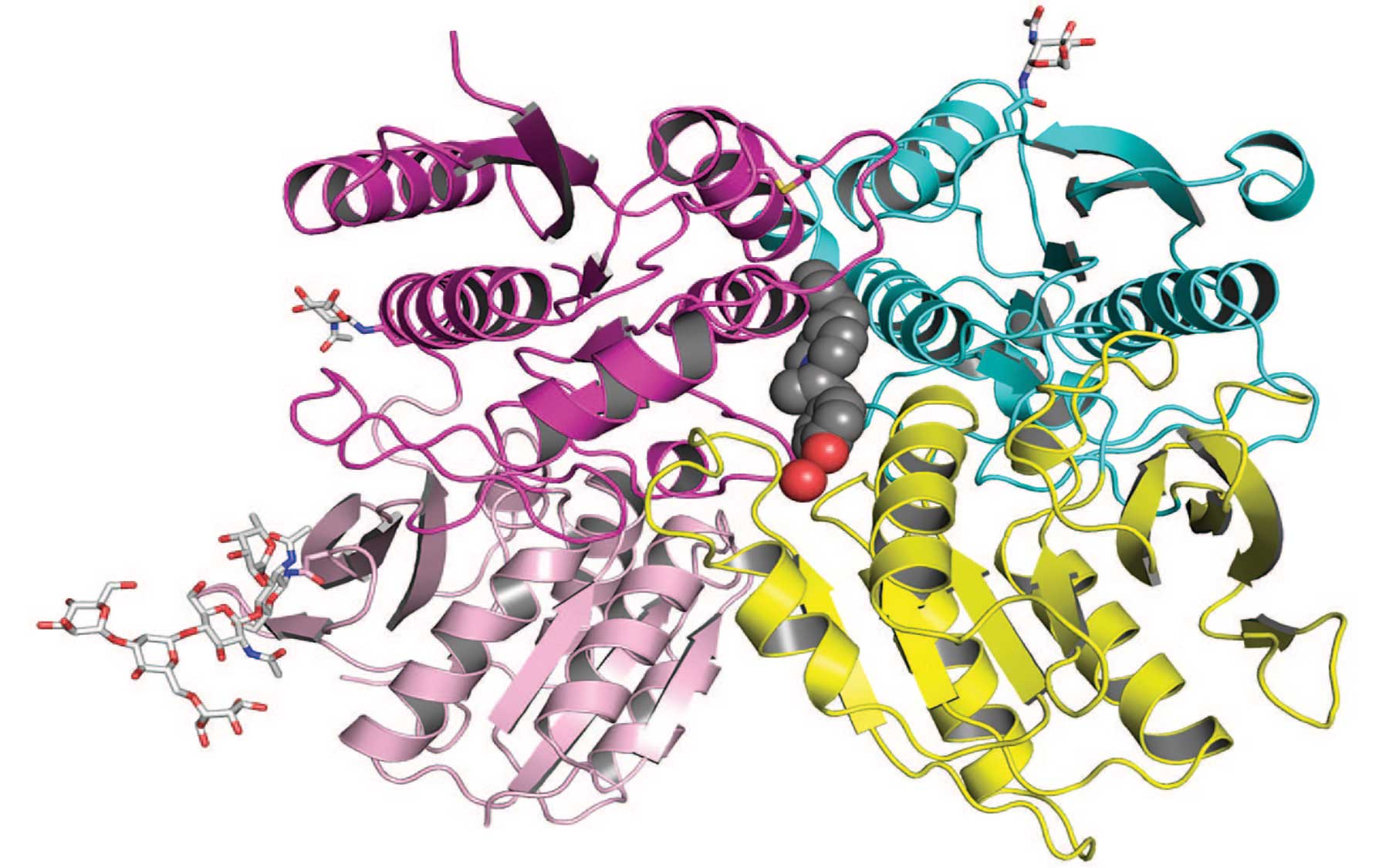
The CSHL team used x-ray crystallography to obtain a very precise map of the structure of the amino terminal domain (ATD) of the GluN1b–GluN2B subunits of the NMDA receptor, including its allosteric binding site. This view, at a resolution of 2.6A˚, shows the subunits docked with ifenprodil (grey spheres at the interface of the two subunits), a known allosteric inhibitor of the NMDA receptor.The newly discovered binding site—a docking port within the receptor—is important because it is a potential target for drugs that can modulate NMDA receptors, dysfunctions of which have been implicated in depression, schizophrenia, Parkinson’s and Alzheimer’s diseases as well as stroke-related brain injuries.
Allosteric sites in receptors are distinguished from their “primary” or “active” binding sites. Importantly, the newly obtained molecular map will enable scientists to design highly specific compounds that home in on the allosteric site, thereby minimizing “off-target effects,” which give rise to a drug’s unwanted side effects.
In a study led by CSHL Associate Professor Hiro Furukawa and published June 15 in the journal Nature, the allosteric site of interest is shown to be in the region of NMDA receptors called the amino terminal domain. A class of allosteric inhibitors for NMDA receptors, called phenylethanolamines, has previously been identified. One such compound, ifenprodil, is known to bind specifically to the GluN1/GluN2B subtype of the NMDA receptor, but not to other subtypes.
The neuroprotective properties of phenylethanolamines have inspired scientists to employ them for treatment of neurological diseases and disorders. Some are now being tested in clinical trials for depression, pain, Parkinson’s disease, and Alzheimer’s disease.
The detailed blueprint of the allosteric site where phenylethanolamines bind to the receptor will facilitate rational design of improved compounds. In the work published on June 15th, Furukawa’s group identifies the precise binding site of phenylethanolamine within the amino terminal domain of GluN1/GluN2B NMDA receptors. The results were obtained through biochemistry and x-ray crystallography, a method that features exposing a crystalline form of the molecule under study to very high-energy x-ray beams, which reveals its features in great detail. This enabled the team to demonstrate that phenylethanolamine is recognized at the interface of the GluN1 and GluN2B subunits of the receptor, rather than at a previously predicted site within GluN2B.
“Before this study, we did not have a sufficiently precise map of NMDA receptor subunits to facilitate the design of better and more effective compounds that could dock at the allosteric site. Our results should move drug development in the right direction. We are now optimistic that the field can determine optimal ways of targeting NMDA receptors for therapeutic purposes,” Furukawa says.
Written by: Peter Tarr, Senior Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
This work was supported by NIH MH085926, the Alzheimer’s Association and a donation from the Fox family. Dr. Furukawa was also funded by a scientist development grant from the American Heart Association. Team member and co-author Erkan Karakas is supported by a NARSAD Lieber Young Investigator Award.
Citation
“Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors” was published online ahead of print in Nature on June 15, 2011. The authors are: Erkan Karakas, Noriko Simorowski and Hiro Furukawa. The paper can be accessed at www.nature.com by using the following identifier: doi:10.1038/nature10180.
Principal Investigator

Hiro Furukawa
Professor
Cancer Center Member
Ph.D., The University of Tokyo, 2001