Discovery explains one of the evolutionary needs to retain the enzymatic activity of the Argonaute2 protein in mammals
Cold Spring Harbor, NY — MicroRNAs are small bits of RNA within cells that wield enormous power. They influence virtually every biological process by controlling the “expression” of genes. Helping them in exerting this control is a unique class of proteins called Argonautes.
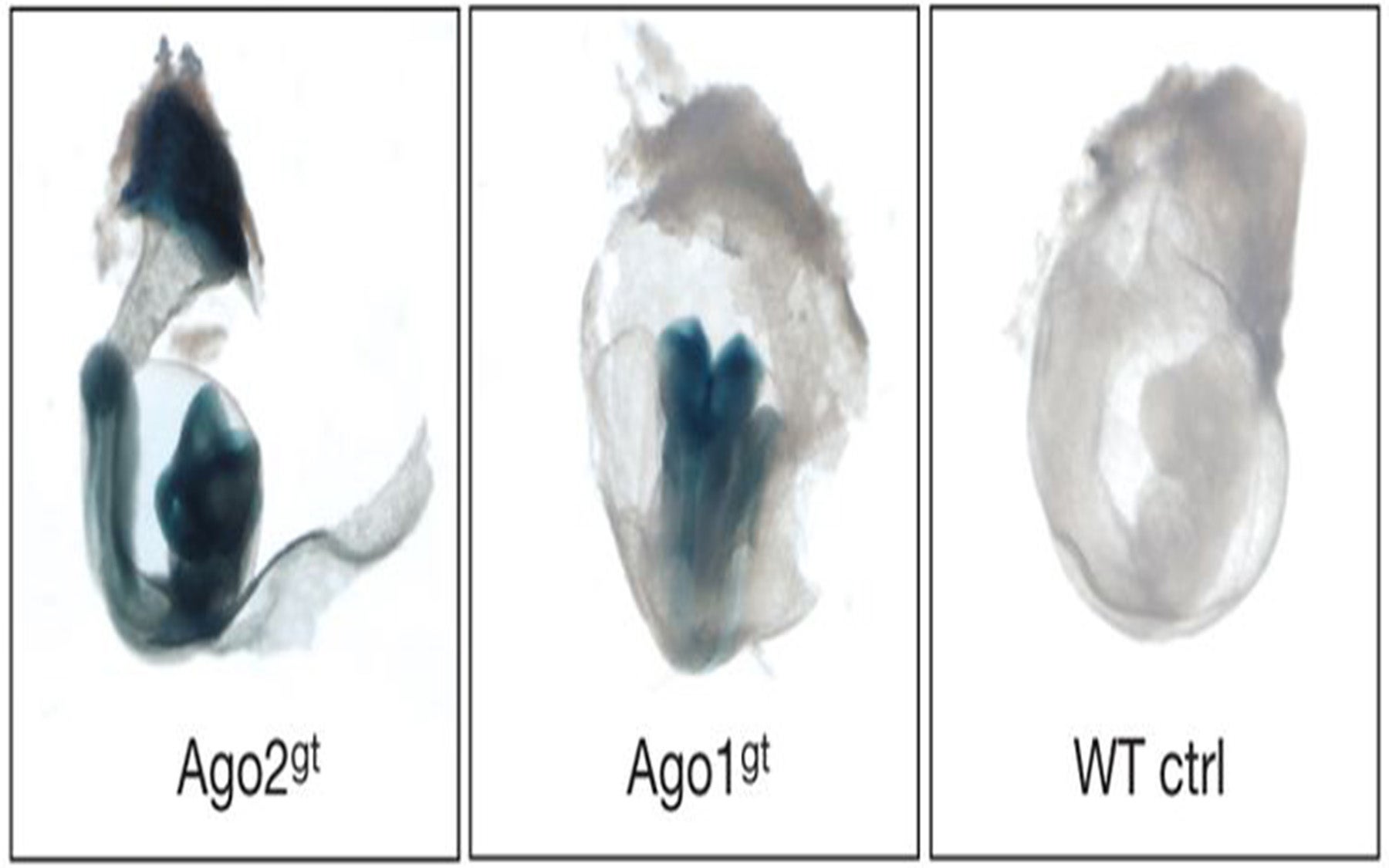
Cold Spring Harbor Laboratory (CSHL) researchers led by Professor and HHMI investigator Gregory Hannon, Ph.D, now report that in animal cells, one of Argonautes, called Ago2, has a different role—it helps generate microRNAs instead. The study, which appears online, ahead of print in Nature on April 27th points to an alternative pathway of microRNA generation that the team discovered by analyzing a microRNA molecule produced in developing red blood cells.
In 2004, a team led by Hannon and Leemor Joshua-Tor, another CSHL Professor discovered that Ago2 functions as a slicer enzyme that chops up its targets—messenger RNAs encoded by genes—by using small RNA molecules (for example, microRNAs) as guides to home in on the correct messenger RNAs. The team’s subsequent studies revealed a possible role for this slicing, or catalytic, activity of Ago2 in mammalian oocytes.
“But we found that although Ago2 is critical for the viability of embryos during development in mice, Ago2’s microRNA partners generally undertake gene regulation during embryogenesis in ways that do not require the catalytic activity of this enzyme” explains Hannon. “So the basis of the evolutionary pressure to retain this catalytic ability was a mystery to us.”
Engineering mice with catalytically inactive Ago2, the team has now found that mice give birth to progeny that die a few hours later, showing symptoms of anemia. “These mutant animals have only 50% of the normal level of red blood cells at birth,” says Sihem Cheloufi, a Stony Brook University graduate student pursuing her doctoral research in Hannon’s lab. “Their lack of catalytically active Ago2 hampers their normal postnatal development.”
Cheloufi and her colleagues have traced a molecular cause of this lethal defect to a single microRNA, miR-451, that’s present in normal mice but missing in the mice that lack catalytically active Ago2. Further experiments showed that the absence of miR-451 in the mutant mice was due not to a defect in its production but the subsequent “maturation” steps that produce a functional microRNA molecule.
Most known microRNAs, which are about 24 “letters” or nucleotides long, are much longer when they are produced and “mature”—or get trimmed down to size—via a two-step process. After the first trimming step by an enzyme called Drosha, the shortened RNA molecule folds on itself like a hairpin.
Normally, the hairpin’s curve, called the “loop” is subsequently cut out by another enzyme called Dicer, leaving behind a double-stranded “stem”. This is the mature microRNA that gets picked up by Ago2. One strand is eventually discarded; the other Ago2-bound strand is ready to seek out and trigger the destruction of its messenger RNA target.
Hannon’s team has found, however, that miR-451 maturation proceeds via a different pathway, where the Dicer step is skipped and the immature hairpin is directly loaded into Ago2. “Ago2 then essentially performs its slicing job to complete the maturation of this microRNA,” Cheloufi explains. The team attributes the ability to Ago2 to bind to the immature miR-451 hairpin to its unique structure that is also highly conserved in vertebrates.
“The existence of this alternative pathway explains some of the evolutionary pressure to maintain a catalytically active Argonaute protein in animals,” says Hannon. His team is now hunting for other RNA molecules that might be similarly generated and that might be critical for normal embryogenesis.
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455
Citation
“A dicer-independent miRNA biogenesis pathway that requires Ago catalysis” appears as an advance online publication on April 27th in Nature. The full citation is: Sihem Cheloufi, Camila O. Dos Santos, Mark M. W. Chong & Gregory J. Hannon. The paper is available at http://www.nature.com/nature/journal/vnfv/ncurrent/abs/nature09092.html