Moses’ need for speed landed him at K. Barry Sharpless’ lab at Scripps Research as a postdoc. “Click chemistry” is the term Sharpless, a Nobel Prize–winning chemist, devised to describe a particular class of fast, reliable, and high-yielding reactions. Click chemists use cleverly designed catalysts to cause pairs of molecules to assemble—click together—like LEGO® bricks. The reactions are not only fast, but they are also specific. And they are changing how cleanly and efficiently drugs and other materials can be made.
CSHL Professor John E. Moses and collaborator Nobel laureate K. Barry Sharpless show click chemistry in action, synthesizing a helical polymer in about 10 minutes.
Fast chemistry
One of the biggest obstacles to making any molecule is the speed of the reaction. Catalysts, including enzymes in biological systems, make slow reactions happen faster. Moses developed click chemistry using sulfur and fluoride exchanges, or SuFEx reactions, plus a catalyst, to link small, versatile molecules to other reaction partners. These click reactions happen very quickly—in some cases, almost instantly.
To make more complex molecules, chemists string together a series of reactions. Moses and collaborators have reengineered production methods for a few important, older molecules like the antibiotic vancomycin. In other cases, they are inventing a broad spectrum of useful new molecules.
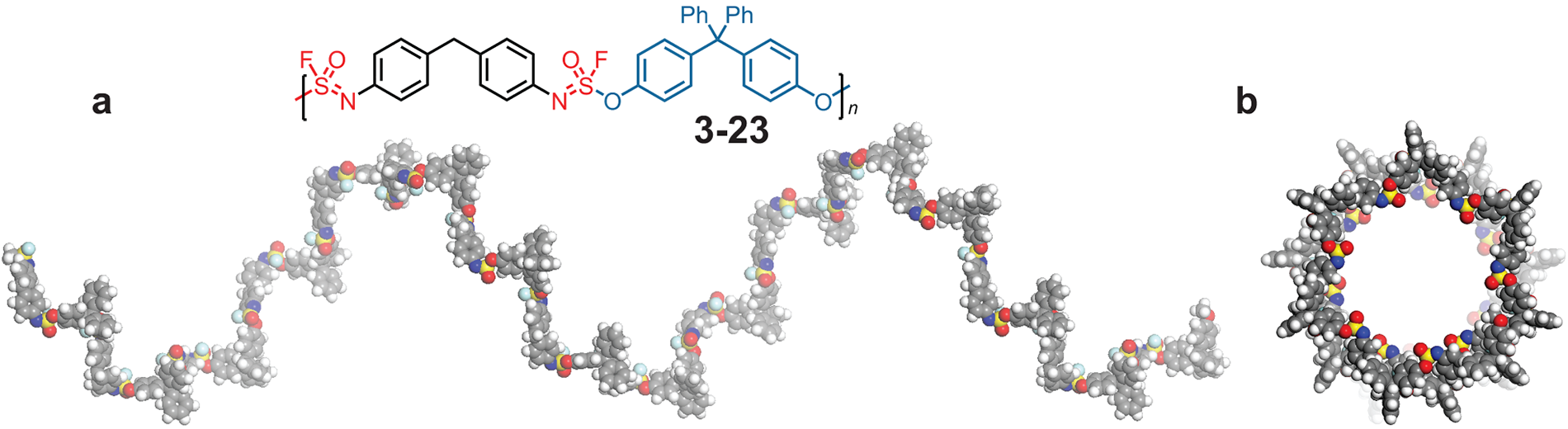
One exciting product is a polymer: long strings of identical units hooked together one after another. Natural polymers include silk, wool, and plant fibers. Plastics and synthetic rubber are synthetic polymers.
Making a non-polymer requires two molecules that fit together; when their pairing is finished, there are no more reaction sites left for that kind of reaction. But add a second reaction site to each molecule (or “monomer”), and the reaction can lead to a string of monomers linked together like a chain. Moses reported in Nature Chemistry on a versatile new polymer his lab and collaborators invented that takes less than a day to make. The team designed monomers with a third reaction site available to add side chains in later reactions, yielding a polymer decorated along its chain like a Christmas tree.
Dirty reactions add up
Click reactions may be fast, but if the reactions do not use up all the starting ingredients, they are full of unwanted side products, or yield few of the right molecules. The time saved in making the reaction will be lost in purifying the product. For simple reactions, a few percentage points of material ending up as byproducts or leftovers, may not constitute a problem. But many drugs require a series of reactions, and leftovers quickly add up. Not only that, but the solvents used to purify desired products can be bad for the environment.
Fortunately, click chemistry is so efficient that most of the ingredients end up making the product and leaving little waste. That is because click reactions are designed to involve very willing reaction partners. The force driving the reaction is so strong that the molecules will nearly always bond the same way, produce the wanted materials, and leave behind little unused or misused reactants. Purifying a product can require organic solvents, but click reactions are designed to work in water or nontoxic solvents, making these reactions less toxic to the environment.
Teaching old drugs new tricks
Moses and other click chemists are developing families of reaction partners. For example, with antimicrobial resistance becoming an increasing problem, Moses and his team are making new antibiotics out of old ones. In 2020, they reported generating nearly 300 new candidates in the journal Angewandte Chemie. They had made small changes to a library of potential drugs—some showed activity against the dangerous drug-resistant bacterial infection MRSA (Methicillin-resistant Staphylococcus aureus).
There are many diseases for which there are few or no treatments available. The Moses lab is working on developing a treatment for one such disease: leprosy. Joshua Homer, a chemist and postdoc in the Moses lab, recently received a $150,000 grant from the New York Community Trust to create new drugs using a rapid click chemistry approach. Moses notes how hard it has been to come up with a good drug “because leprosy is very slow-growing. It takes a long time to kill it because it doesn’t replicate very fast.” That means people with the disease need to take a drug for a long time. Homer says, “drug treatments that are available have some pretty severe side effects, like skin discoloration for one. Hopefully, we’ll get some derivatives that are better at treating leprosy and reducing some of those side effects.” They designed 200 new versions of three previous leprosy drugs and are currently testing them for efficacy.
Moses hopes to keep teaching old drugs new tricks. He is excited to develop new drugs with his biology colleagues at CSHL, saying:
“We bring materials that they [biologists] can’t get, they can’t access. It’s as simple as that. Things that have never been created in the universe before. They look pretty, but what do they do? How do you find out? We can give them to a biologist, who has an anti-microbial assay, or a cancer expert who has a cell-based assay.”
With back and forth between large libraries of chemical tweaks and cutting-edge biological approaches, CSHL is poised to invent and test many new molecules which may have therapeutic applications.
Editor’s note
John E. Moses joined the CSHL faculty in 2020 as the first chemist in the Laboratory’s 130-year history. He did his Ph.D. (DPhil) at the University of Oxford. Moses began working on click chemistry as a postdoc in Nobel laureate K. Barry Sharpless’ lab at Scripps Research. The chemistry between Sharpless, Moses, and the click chemistry community seems pretty unstoppable. For their innovative work, the group won the first-ever Organic Division Horizon Prize: the Robert Robinson Award in Synthetic Organic Chemistry from the Royal Society of Chemistry.
Written by: Luis Sandoval, Communications Specialist | sandova@cshl.edu | 516-367-6826