SOF4 is a gas that was discovered over a hundred years ago but is rarely used because it is difficult to prepare and highly reactive. Now a collaboration of chemists including Cold Spring Harbor Laboratory (CSHL) Professor John E. Moses, Nobel laureate K. Barry Sharpless and Associate Professor Peng Wu, both of Scripps Research, and Han Zuilhof of Wageningen University found a way to use the molecule safely as building blocks for new products. In a paper in Nature Chemistry, they describe a new set of modifiable polymers made from SOF4.
It was a team effort to tame the molecule. Zuilhof says, “The goals of the various research groups were different, but we combined all our expertise to achieve our many goals.” Suhua Li, the lead author of the study and a former postdoc in Sharpless’ lab, started with a tiny container of the gas in the lab, but after he used that up, he says, “there was no more SOF4 available anywhere in the world. The great potential of the SOF4 chemistry inspired me to make the gas by myself, even though the procedure seemed dangerous.”
This is the first in a series of videos about the cool science tools used by scientists every day at Cold Spring Harbor Laboratory (CSHL). CSHL Professor John E. Moses is a chemist whose laboratory synthesizes novel molecules using a form of green (relatively efficient and clean) chemistry, called click chemistry. His team discovers new materials, therapeutic drugs, and tools for biology. CSHL postdoc chemist Joshua Homer demonstrates how the Moses lab purifies molecules they synthesize using the rotating evaporator. The machine, which they usually call the “rotovap”, can separate a mixture of two different chemicals, removing a more volatile liquid from a less volatile liquid or solid. Alternatively, the machine can remove all solvents from a solution, leaving only the solids behind. The machine applies heat and vacuum to the system, keeping the temperature even by rotating the round container. The liquid chemical with the lower boiling point evaporates from the first container. The evaporated vapor condenses onto a cooled, twisted coil and drips into a collection container below. What is left behind in the first container is what the scientists were trying to purify.
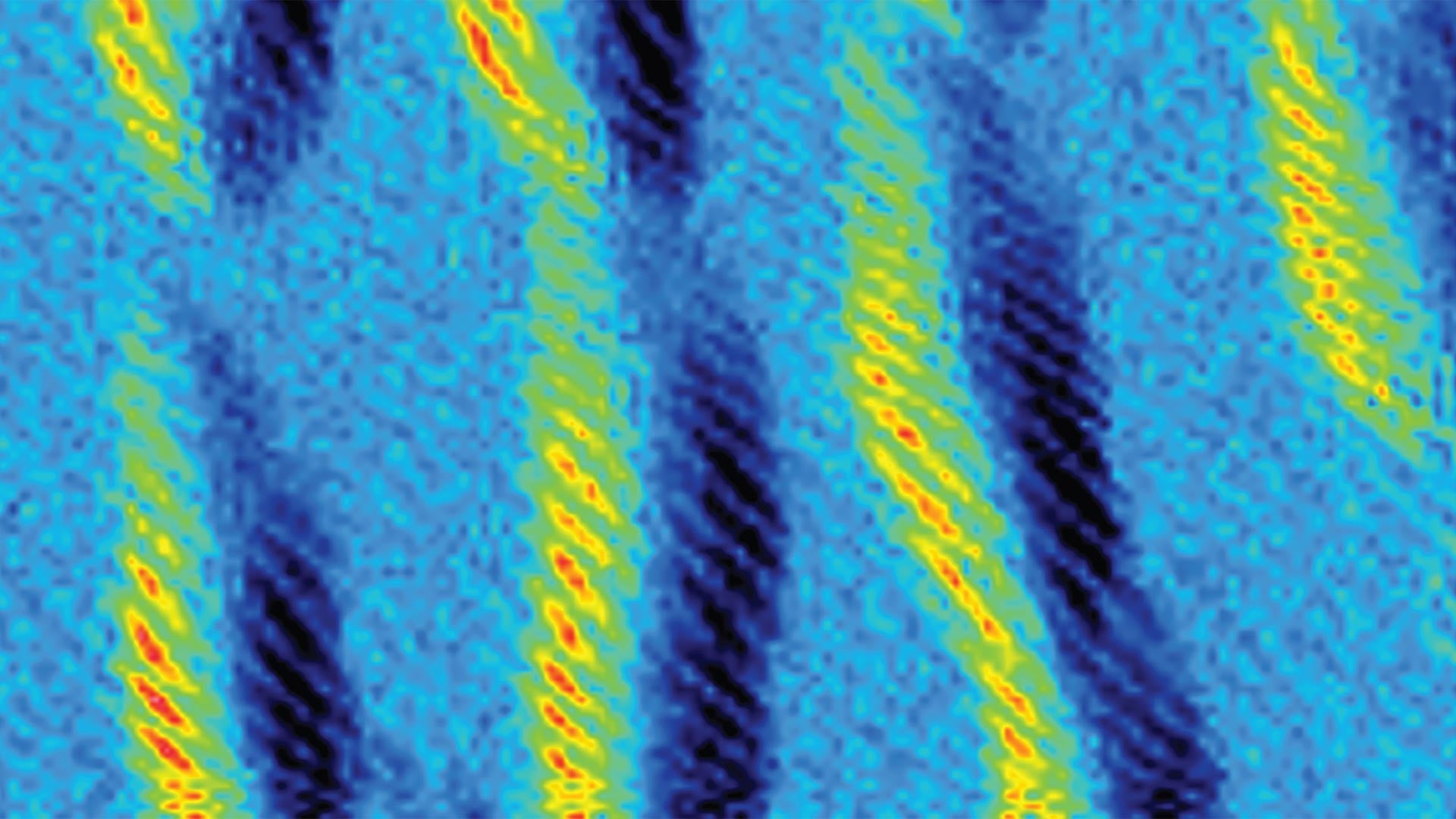
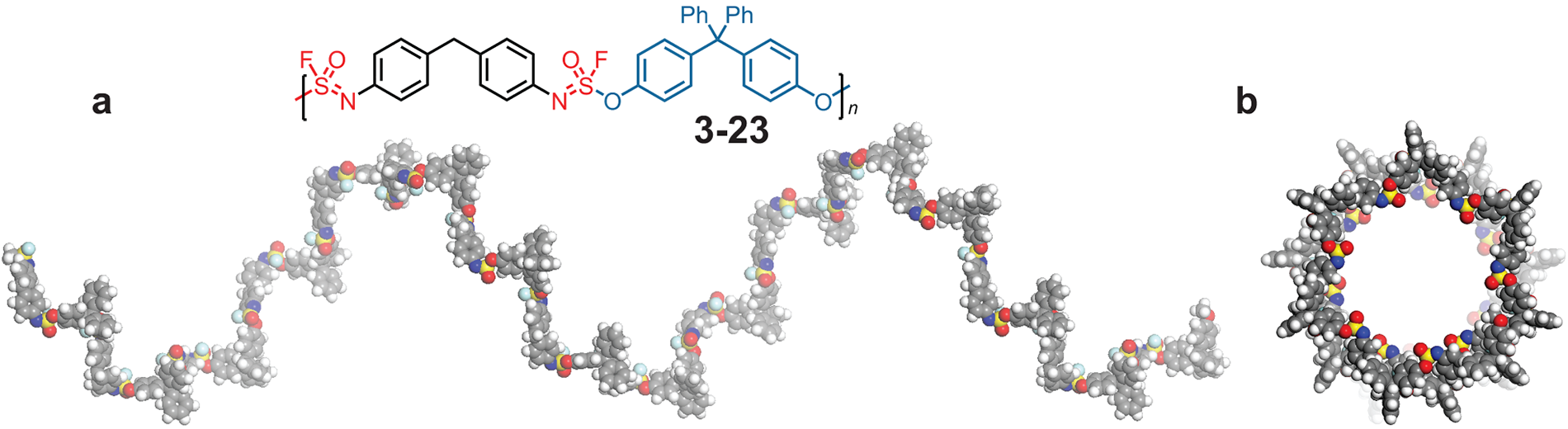
The SOF4 molecule could be used as a hub to link together diverse components into a modular family of new—and potentially valuable—materials. Even more importantly, the scientists found that the reactions could be done in an environmentally safe way without dangerous solvents and polluting byproducts—a form of what chemists call “click chemistry”.
Click chemistry allows users to “click” together two molecules with precision, speed, and reliability. The team used SOF4 to form long chains—polymers—with room for important modifications. Moses says:
“That’s why click chemistry is great, really! These polymers could be made in one day. As long as we have the gas, we could do all that chemistry in one day, make a polymer, and post-modify it in one day. That’s incredibly fast.”
This new chemistry will allow scientists to generate a vast new library of polymers, each with its own distinct properties and applications in drug discovery and material science. Sharpless says, “It is the making and breaking of the nascent, growing polymer links that let us access what seems magical.” Moses adds:
“The opportunity for these polymers, I think, is infinite. There are so many things we can do with it, really. We’re limited by our imagination.”
Written by: Luis Sandoval, Communications Specialist | sandova@cshl.edu | 516-367-6826
Funding
National Science Foundation, National Institutes of Health, Australian Research Council for Supporting Future Fellowship, Guangdong Natural Science Funds for Distinguished Young Scholar, Program for Guangdong Introducing Innovative and Entrepreneurial Teams, Pearl River Talent Recruitment Program, National Science Foundation of China, King Abdulaziz University, Office of Science, Office of Basic Energy Sciences of the US Department of Energy
Citation
Li, S., et al., “SuFExable polymers with helical structures derived from thionyl tetrafluoride”, Nature Chemistry, August 16, 2021. DOI: 10.1038/s41557-021-00726-x
Principal Investigator

John Moses
Professor
Cancer Center Member
Ph.D. (DPhil), University of Oxford, 2004