Study Reveals Key Properties of Silent versus Active DNA
Until recently, proteins called “histones” have been the Rodney Dangerfield’s (“I don’t get no respect!”) of the biochemical world. Histones bind to DNA and wrap the genetic material into “beads on a string” in which DNA (the string) is wrapped around small blobs of histones (the beads) at regular intervals. In the past, many researchers believed that histones played a passive role in chromosome architecture and little role in specifically switching genes on and off. Now, scientists who remained true to histone research are basking in the glow of renewed respect for the dynamic role that these proteins are being found to play in the inheritance of specialized chromosome structures and the control of gene activity. Moreover, evidence suggests that defects in the establishment of proper chromosome structure by histones may activate or silence genes aberrantly and thus lead to disease.
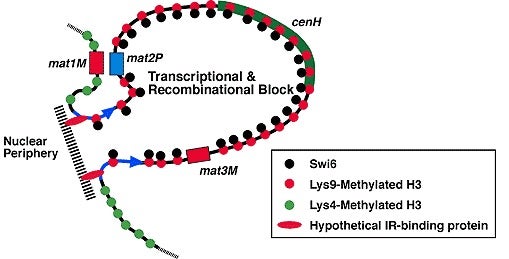 In the August 10 issue of Science, Cold Spring Harbor Laboratory researcher Shiv Grewal and his colleagues report that seemingly small differences between two varieties of histone have dramatic effects on chromosome structure and gene expression. They found that “silent” regions of chromosomes—where genes are kept “off” and DNA resists genetic recombination—contain one variety of histone H3. In contrast, the researchers found that “active” regions of chromosomes—where genes can be easily switched “on” and DNA can readily recombine—contain a slightly different variety of histone H3. Histone H3 in silent DNA had a “methyl” group attached to a particular lysine amino acid, #9. Histone H3 in active DNA had a methyl group attached to a different, nearby lysine amino acid, #4.
In the August 10 issue of Science, Cold Spring Harbor Laboratory researcher Shiv Grewal and his colleagues report that seemingly small differences between two varieties of histone have dramatic effects on chromosome structure and gene expression. They found that “silent” regions of chromosomes—where genes are kept “off” and DNA resists genetic recombination—contain one variety of histone H3. In contrast, the researchers found that “active” regions of chromosomes—where genes can be easily switched “on” and DNA can readily recombine—contain a slightly different variety of histone H3. Histone H3 in silent DNA had a “methyl” group attached to a particular lysine amino acid, #9. Histone H3 in active DNA had a methyl group attached to a different, nearby lysine amino acid, #4.
Interestingly, Grewal and his colleagues showed that deleting “boundary elements” which mark the transition between silent and active regions of DNA allowed the spreading of lysine 9-methylated histones into neighboring DNA normally occupied by lysine 4-methylated histones. This finding has important implications for many types of genetic disease. It indicates that damage to a boundary element in a critical region of a chromosome could lead to abnormal silencing or the switching off of genes in that chromosomal region, with potentially serious consequences.
David Allis (University of Virginia), Grewal’s colleague in this and another study published earlier this year in Science, has proposed that in addition to the now-familiar genetic code of repeating As, Cs, Gs, and Ts in the sequence of DNA, a “histone code” exists in which differentially modified histone proteins can organize the genome into active and silent regions that can be stably inherited.
Indeed, working at the National Cancer Institute in 1996, Grewal and former Cold Spring Harbor Laboratory scientist Amar Klar showed that active and silent chromosomal states can be stably inherited through mitosis (cell division) and, remarkably, through meiosis (the production of gametes such as egg and sperm). In essence, Grewal and Klar found that the Mendelian inheritance of traits sometimes depends not only on the faithful replication of DNA sequences but also on the transmission of “higher-order” chromosome structure. In a study published in journal Cell last year, Grewal and colleagues went on to show that the “gene” in these instances is not only DNA, but DNA plus associated proteins. The scientists proposed a chromosome replication model in which both the DNA molecule plus higher-order chromosome structure are duplicated.
The new study in Science is one of several that are beginning to elucidate the role of histones and other proteins in such a chromosome replication process.
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455