Study shows how Vpx facilitates reverse transcription in simian virus life-cycle
Cold Spring Harbor, NY — A team of scientists at Cold Spring Harbor Laboratory (CSHL) has discovered new details about how a simian strain of the AIDS virus replicates. The findings are significant because they suggest new strategies to prevent replication, and because they are applicable to human strains of the virus, which, despite the persistent efforts of scientists over two decades, can only be slowed by drug treatments but neither cured nor prevented.
Jacek Skowronski, Ph.D., CSHL associate professor, led a team that studied a virulent strain of simian immune-deficiency (SIV) virus called SIVsm/mac, named for two species of monkeys in which it occurs, sooty mangabeys and macaques. The team included members of Dr. Skowronski’s CSHL lab and researchers at Stowers Institute for Medical Research in Kansas City, Missouri, and the Skirball Institute of Biomolecular Medicine in New York City.
Like versions of the virus that occurs in humans, SIV viral particles, or virions, are composed of a few fundamental parts. At their heart are two identical but separate strands of RNA surrounded by a protective envelope of roughly 2,000 proteins called a capsid.
This tiny, conical capsule, in turn, is surrounded by multiple defensive rings, somewhat like the walls of a medieval city. Immediately surrounding it is a protective protein shell, or matrix, and beyond it a formidable double-walled viral envelope. Poking through the outer envelope are the viral equivalent of grappling hooks, protein molecules designed to lock onto receptors on the surface of the unfortunate cell that the virus will attach to and then invade.
Viruses Hijack Living Cells to Reproduce
Viruses, unlike cells, are not living things. They must penetrate a living cell and commandeer its internal machineries in order to reproduce. HIV and its simian cousin SIV are members of a viral subspecies called retroviruses that invert the usual reproductive procedure. Their genetic raw material is not DNA but rather RNA, and before they can begin to replicate, they must first convert their RNA into DNA, using a special enzyme that they encode, called reverse transcriptase.
Once its RNA has been reverse-transcribed into DNA, the virion, having invaded a cell whose genetic material consists of DNA, can shed its protein coat and immediately proceed to integrate its newly converted DNA—containing 9 genes—into the host cell’s DNA. In this way the virion effectively hijacks the cell and reproduces itself whenever the cell reproduces.
Dr. Skowronski has devoted years to the study of various molecular factors—think of them as assistants—that immune-deficiency viruses employ to perform a range of essential tasks. The idea behind his approach is to understand with great precision all of the details of the processes by which the virus lives and propagates, as a means of identifying points of vulnerability, where drugs might be inserted to foul up the works.
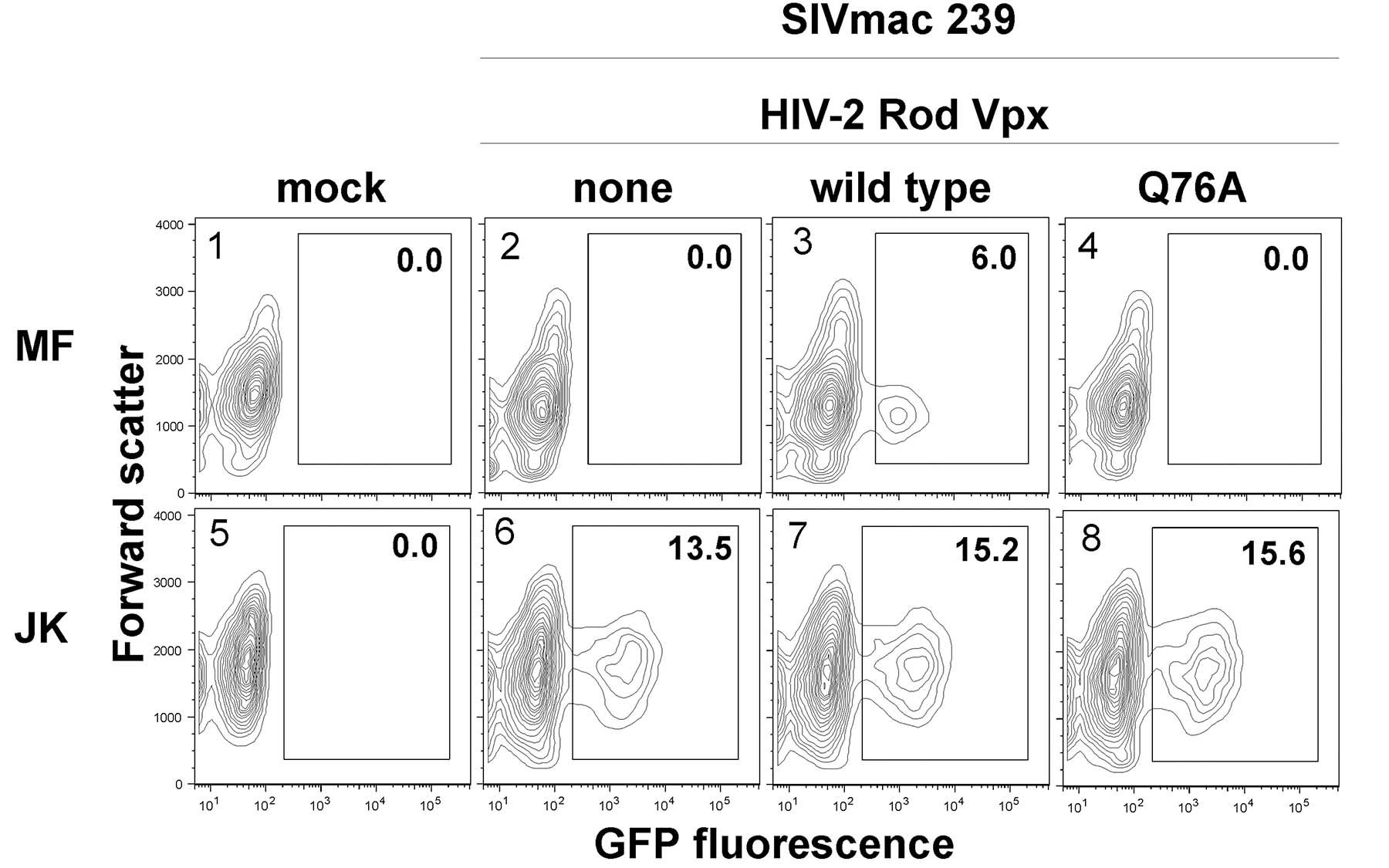
In the research just completed, results of which appeared in PLoS Pathogens on May 9, Skowronski and his team focused on a so-called accessory protein called Vpx (Viral protein x). Prior studies had shown that Vpx was produced by simian, as well as a subset of human, immunodeficiency viruses, and was somehow active at the heart of their reproductive processes in a subset of immune cells called macrophages. The question was how, and to what effect.
How Vpx Enables the Virus to Replicate
Macrophages are central players in the mammalian immune system. Immune-deficiency viruses are devastating because they specifically seek out, invade, and commandeer the machinery of these particular cells—macrophages, dendritic cells, helper T-cells—which protect the mammalian system from foreign invaders.
Skowronski and colleagues knew from prior work that Vpx was a key enabler: it somehow facilitated an “early event” in the viral life cycle that helped the virus invade macrophage target cells. Recent studies had further shown that Vpx proteins in both monkey and human viruses promoted the process of reverse transcription that underlies the conversion of viral RNA to DNA.
In their study, which consisted of several steps, the CSHL team showed that when the vpx gene (the gene that encodes the Vpx protein) was deliberately deleted, the virus went about reverse transcription “very inefficiently.”
“This suggests that the Vpx protein is key to the process by which the virus infects macrophages,” Dr. Skowronski comments, “and further, that it seems to be acting either before and/or during the reverse transcription process.” This new view of Vpx’s role contrasts with a prior hypothesis that it was involved in the transporting of genetic material that had already undergone reverse transcription.
The team’s experiments revealed that the Vpx protein in the SIVmac virus binds to a complex of three cellular proteins that in turn engage molecular machinery involved in the degradation of proteins. Thus, the team revealed for the first time not only that Vpx interacted with this system—called the ubiquitin-dependent proteasomal protein degradation mechanism—but also identified precisely the way it does so, via a series of intermediate steps.
“The net result,” says Dr. Skowronski, “is that we show how Vpx enables efficient reverse transcription in the simian virus, and in so doing, overcomes an innate block that otherwise prevents viral replication.”
By implication, this suggests a strategy by which a future drug might interfere with the reproductive machinery of the virus to prevent or limit its ability to spread. “There are no guarantees, of course, that such an approach will work,” Dr. Skowronski says, “but unless we understand molecular mechanisms such as this one that empower this remarkable virus, we are not likely to devise a means of stopping it.”
Written by: Communications Department | publicaffairs@cshl.edu | 516-367-8455
Citation
“Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for Cullin 4 E3 ubiquitin ligase to enable macrophage infection” appeared in Public Library of Science – Pathogens on May 9, 2008. The complete citation is: Smita Srivastava, Selene K. Swanson, Nicolas Manel, Laurence Florens, Michael P. Washburn, Jacek Skowronski.
The paper is available online at:
http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000059