An unusual, but beautiful sight can be found among the figures of a recent scientific paper about some of the tiny machines in our brain: watercolor paintings by Annabel Romero Hernandez, who just graduated from CSHL’s Watson School of Biological Sciences.
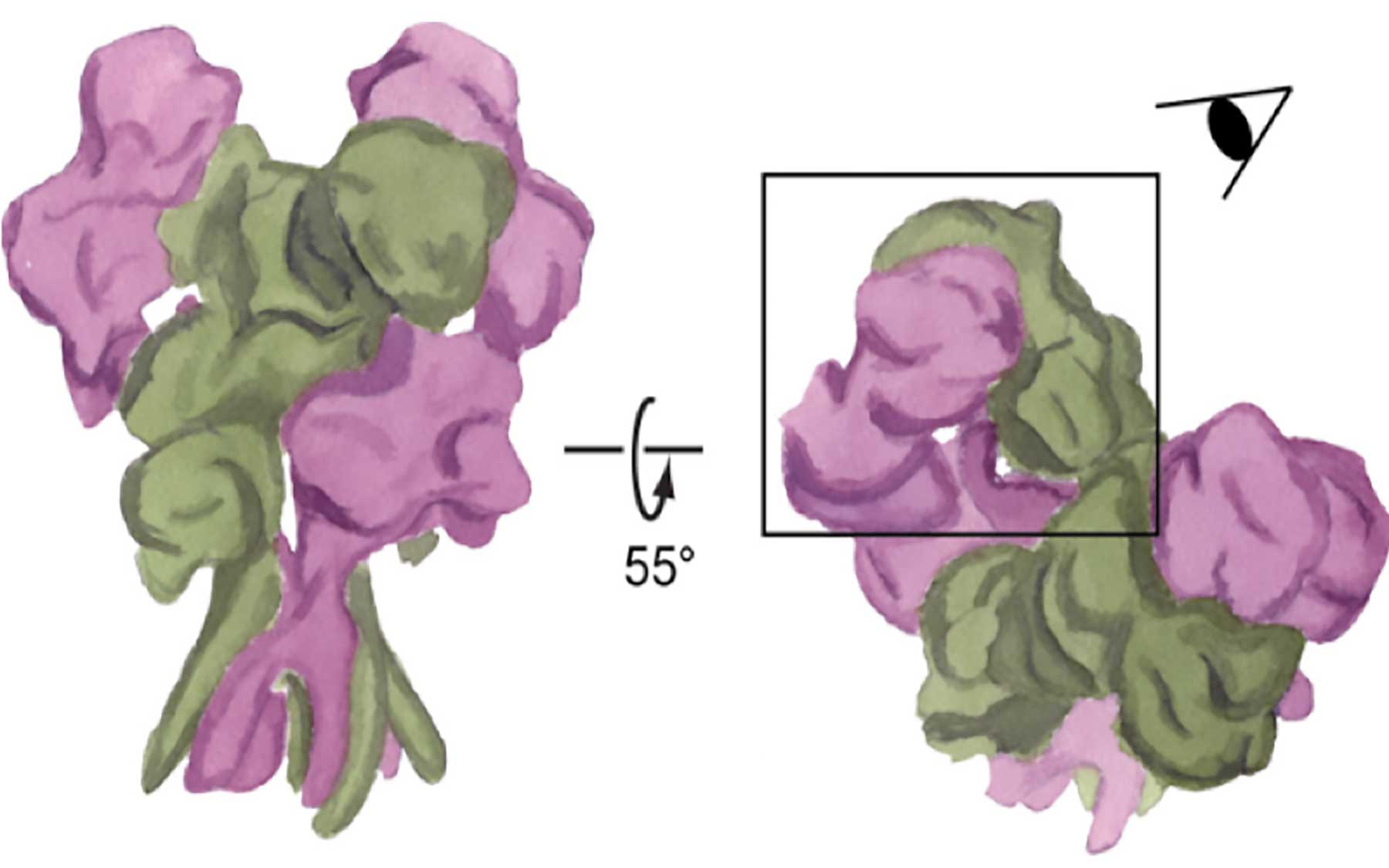
Staring intently at a watercolor painting of what might be mistaken for a bouquet of flowers, the head of my lab asked, “Could you make a figure to represent the proteins?” We were preparing to include the painting in our latest paper and submit it to an academic journal. That meant my artistic representation of an NMDA receptor—a molecule critical to learning and memory—had to be scientifically correct.Creating watercolor art for a scientific paper isn’t as absurd as it might sound. Artistic paintings are often filled with specifics that are only perceived when analyzed carefully. Since I started my painting journey, all those tiny details that we normally ignore in our daily lives became more obvious, and I started appreciating things in a different way. Something similar happened to me through the course of my Ph.D. research. As a structural biologist, my main passion-feeder is discovering the details in the structure of large molecules like proteins: How do proteins do their jobs? What is the blueprint of these tiny machines?
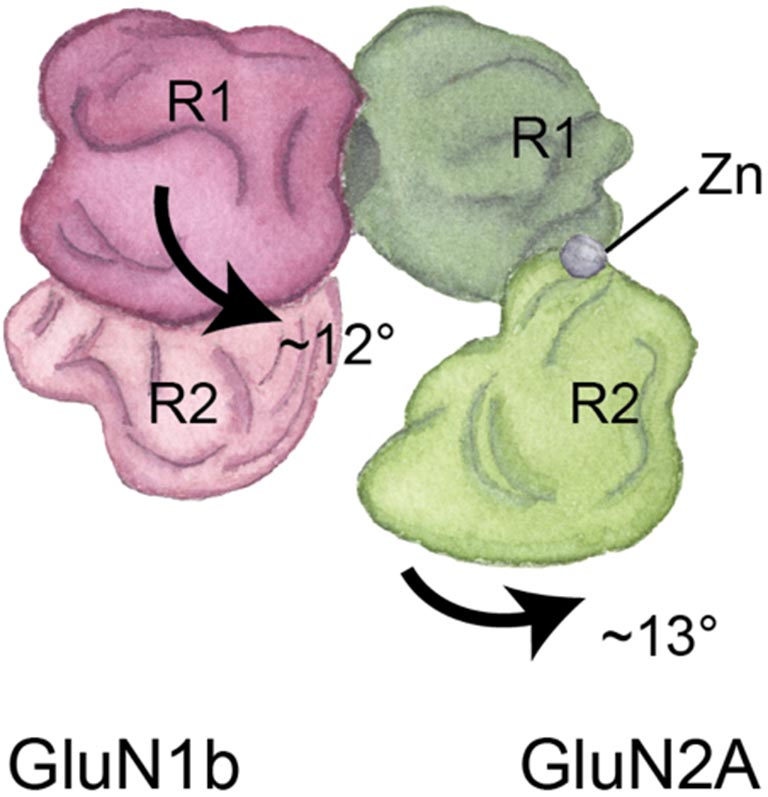
It was natural to combine my artistic and scientific perspectives. After I used X-ray crystallography—the most commonly used method to visualize details at the atomic scale—to discover a new structure within NMDA receptors, I needed to explain the differences between our novel structure and previous findings. My painting wasn’t as detailed as the X-ray crystallography images, but this different medium was ideal for showing the particular details we cared about.
That’s what excites me about the imminent installation of cutting-edge cryo-EM technology at Cold Spring Harbor Laboratory: it will allow me to look at the NMDA receptor differently. Cryo-Electron Microscopy, or cryo-EM, is a technique that visualizes frozen protein samples at cryogenic temperatures—super-cold, under negative 140 degrees Celsius. Unlike X-ray crystallography, which gives you a static picture of a protein, cryo-EM has the advantage of freezing these tiny machines while they’re in action. It works by recording images of thousands of identical, randomly oriented protein molecules. The images are later grouped, aligned and averaged to model a 3D structure of the protein.
When we learn and create memories, the NMDA receptor acts as an important gate to our neurons. It opens and closes to let in or keep out molecules involved in these brain functions. We need to visualize this movement in order to grasp what happens to the receptor when it fails to operate properly in different neurological diseases, and cryo-EM makes this possible.
Cryo-EM could be the revolutionary technique that will take over the structural biology field. However, a more practical strategy is to use X-ray crystallography and cryo-EM together to determine mechanisms of protein complexes. For example, when painting I often use both watercolors and color pencils. I use watercolors to determine the shape of my painting and the shades of color, while I draw tiny details with color pencils. A similar analogy can be used for structural biology methods like cryo-EM and X-ray crystallography.
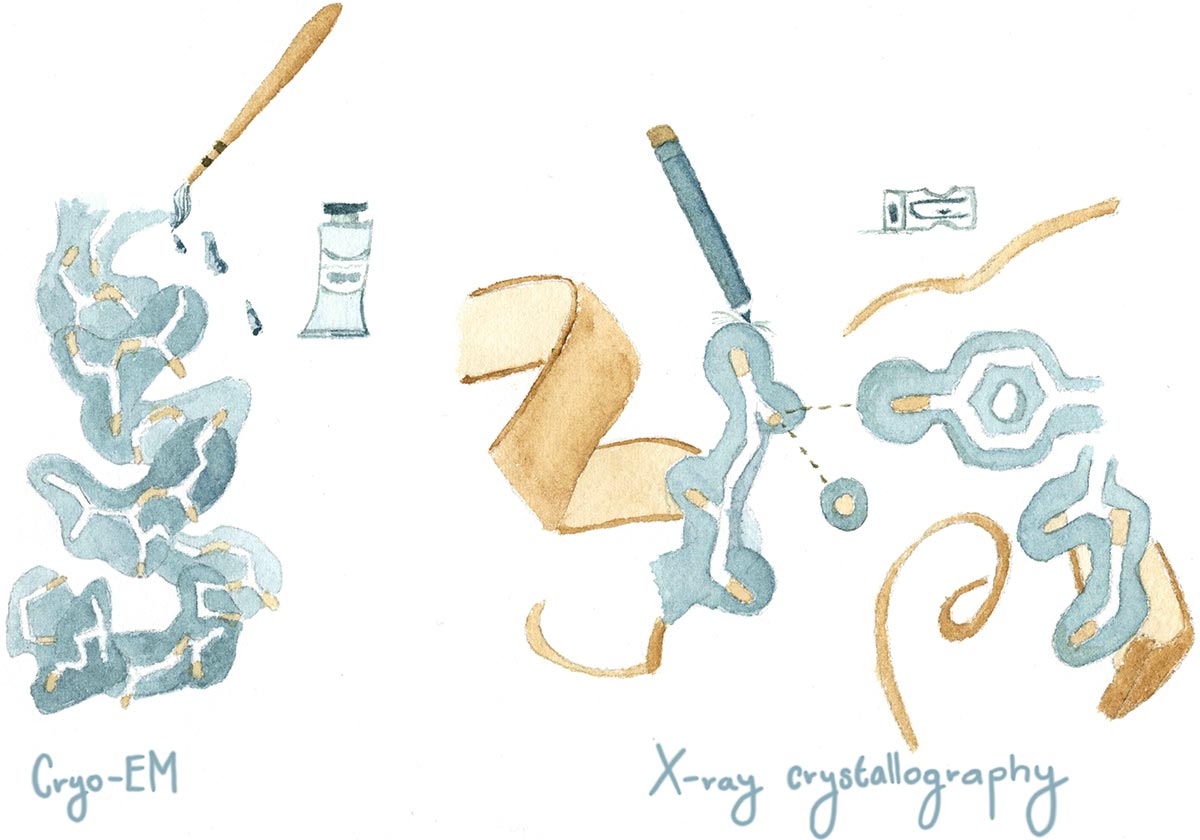
Although this technique is not new—it’s been around for over 35 years—new advances in software and camera detectors have made it more useful. While cryo-EM information can provide insights on the different functional states of the protein, X-ray crystallography can still provide details of atomic interactions with the protein. Putting together pieces of information coming from both techniques will definitely give a broader picture to many of the structure-function questions that remain unanswered today, helping us to more completely understand the masterpieces of form and function that nature through evolution has provided.
You find more of Annabel’s writing and art on her blog, Opuntia Visual.