Killer disease,
Miracle drug
The story of a child’s illness, a scientist’s quest, and the circumstances that made possible the successful development of a life-saving treatment, a miracle drug.
Chapter 1
"Am I just going to watch my child die?"
It’s not a question any mother wants to ask herself.
When Dianne Larson gave birth to her daughter, it was a joyous occasion. In fact, the entire first year was joyous. She was learning to be a mother, and little Emma was just beginning to develop the innocent wanderlust of a newborn. She would lift her head and explore the room with startling amber eyes. Right on schedule, she began to crawl—thrusting herself into a new world full of curiosities.
“At her 12-month pediatrician appointment she was moving her legs, beginning to bear weight on them, as kids are supposed to do. They said, ‘She’s great, she’s perfect. Take her home.’”
“That was 12 months,” Dianne recalls. “At 13 months…that’s when all hell broke loose.”
It took her legs overnight
That’s what it felt like, anyway.
“You feel like the rug is ripped out from under you with this disease,” Dianne explains.
A native New Yorker, Dianne describes herself as an “open book”—not the kind of person to hold back when she feels something is wrong. After seeking the opinion of numerous doctors and experts concerning her daughter’s deteriorating condition, tests were taken. The result? After little more than a year in this world, little Emma Larson was diagnosed with Spinal Muscular Atrophy (SMA)—a disease infamously known to be the deadliest genetic disease among infants. The most seriously affected are often gone before their 2nd birthday.
“Her diagnosis was. . .pretty grim,” Dianne says, wiping the tears from her eyes.
Even with it now all in the past, the emotions from the hardest time in her life still easily bubble to the surface. What happened next, from Dianne’s point of view, was a miracle. In reality, however, it was the fruit of tireless and exemplary science—with all the right people coming together in all the right ways to achieve something wonderful.
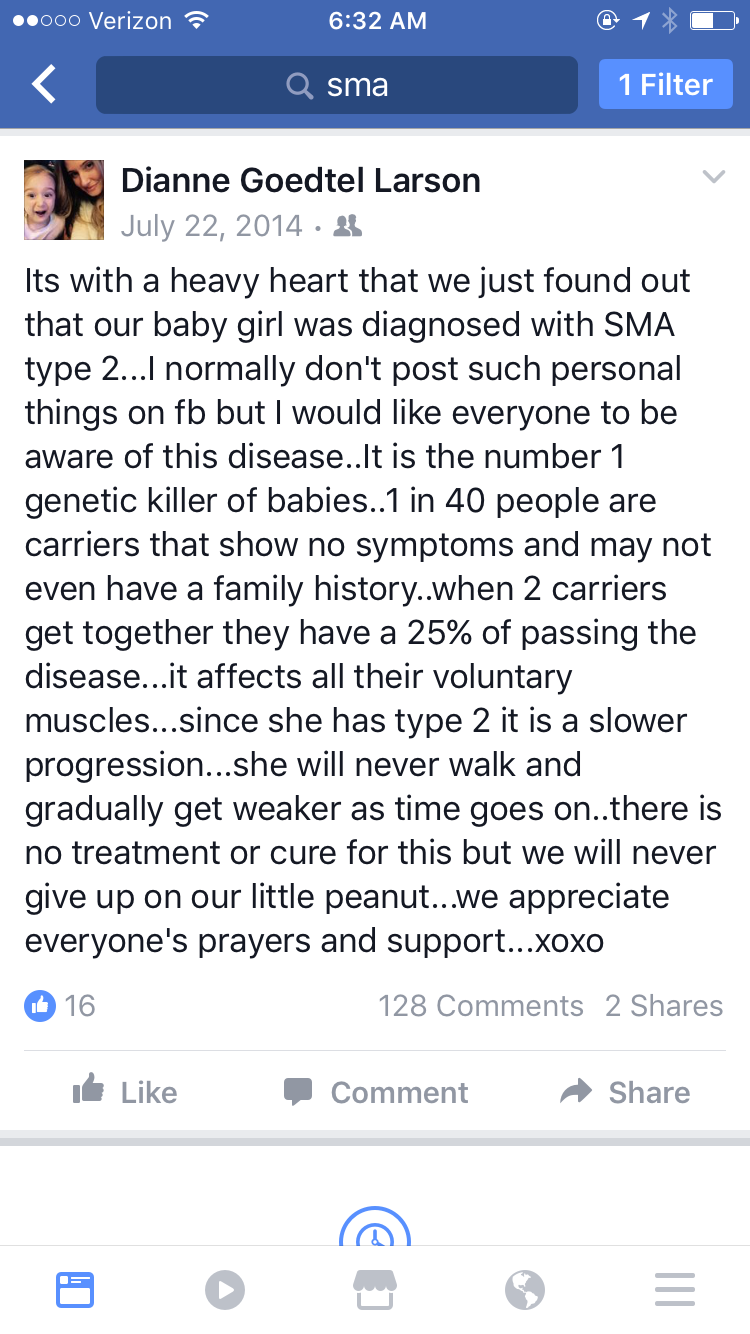
It started with a Facebook post
The first week after Emma’s diagnosis was particularly hard for the Larsons. Dianne and her husband Matt had the unenviable task of informing family and friends about their daughter’s disease.
“It was pretty devastating to keep repeating it.”
Matt places a hand on his wife’s shoulder as she goes on to explain that eventually they decided to inform their friends through social media. It was with that decision, they say, that serendipity then took the reins.
"My husband’s cousin saw the post, and then a friend commented that she worked with a doctor who was working on a treatment for Spinal Muscular Atrophy."
Matt and Dianne took the first opportunity they could to visit Cold Spring Harbor Laboratory, a major research facility near their Long Island home. There they met Dr. Adrian Krainer—a scientist who had been working toward understanding and coming up with a treatment for SMA.
Meeting Dr. Krainer
“Meeting doctor Krainer was like a blast of fresh air,” Matt recalls. “It was so relieving to meet someone who not only understood what was happening to our little girl, but was working to help. He gave us hope.”
Krainer also gave them peace of mind. After doing their own research and speaking with the right people, the Larsons had determined that Emma could be an ideal candidate for Nusinersen—an experimental treatment for SMA that was in the middle of clinical testing. However, they had been hesitant about moving forward. As the inventor of the drug in question, Krainer was able to explain how the drug would work to help their ailing little girl. The Larsons didn’t hesitate to trust him.
"Enrolling your child in a clinical trial... it’s tough,” says Dianne. “It’s very tough on a parent because I don’t want her to hate me for making the wrong decision. You know?...but at this point I feel like we made the best decision for her because she’s doing great."
“If every parent said no...”
Emma’s first injection in the nusinersen trial occurred in March 2015. At the time, Dr. Krainer’s words had left Dianne cautiously hopeful, but she and her husband Matt didn’t expect a miracle.
“She’s doing better than great”
“I even have it written down,” Dianne explains now, over the phone. The sound of papers shuffling crackles over the speaker. “Here it is. She went through all the screenings. Passed them. She had her first injection...I think it was March 3rd. Second injection was at the end of March. Third injection was in May.”
She pauses for a second, then presses on excitedly.
“By this time...This was an amazing thing. It was in April—after the second shot, before her third. I was in the bedroom and she was in the den. Now, mind you she can’t move. She couldn’t crawl more than two feet, if that. She was talking and all of a sudden I hear her voice getting closer and closer.”
Dianne knew Emma had done something—she couldn’t think of what—to make her tiny voice carry across the house. “Emma?” she called out.
“Next thing I know, she’s right by the bedroom door, crawling on the floor. I’m like, ‘What?!’ I was freaking out. I couldn’t believe it. She had crawled from the den to the bedroom! I picked her up and put her back in the den and said ‘Okay, come find mommy again.’ I just couldn’t believe it.”
Emma did find mommy again. And again. It had been just over a month since she had begun the experimental treatment.
An amazing moment for Emma
Faith restored
A religious woman, Dianne admits that when Emma was first diagnosed with a potentially deadly condition, she started to doubt.
“You kind of lose faith, like, ‘Why is this happening?’ I stopped praying, so…when this medicine came, it changed.”
When she and Matt revisited Dr. Krainer and his lab in the summer of 2016, they were all smiles and hugs. Little Emma was a bundle of energy, scooting around in her wheelchair faster than Matt could keep up. With a smile on her face, Dianne explained that these days, she’s praying again, but not for your run-of-the-mill miracle.
"You do pray, I pray for these four people," she adds, gesturing toward Dr. Krainer and the people in his lab. “Because without them there wouldn’t be any hope for my daughter...so they are a miracle for us.”
By June she was standing
Dianne calls it “cruising.” According to the proud mother, Emma braced herself against an ottoman to stand. Then she sidestepped around it in order to reach different parts of the den. She did this without leg braces, opting to stand entirely on her own budding strength.
It’s important to note that before the trial, Emma had never stood. SMA struck too early—before the average newborn takes her first steps.
And now she was standing.
“Doctors haven’t put a limit on it,” Dianne adds. “At this point, we’re just waiting to see how far she can go!”
Chapter 2
A gift from science
It was a gift to the world. On December 23, in the midst of 2016’s holiday season, the drug officially known as nusinersen—commercially branded as SPINRAZA™—was approved by the US Food and Drug Administration. Some went so far as to call it a “miracle drug”—the result of “perfect” fast-tracked clinical trials.
Dr. Darryl De Vivo of Columbia University was involved in trials for nusinersen from the start. He was a prominent player in the drug’s testing and witness to the miraculous recovery of patients much like Emma Larson and Cameron Harding—children who previously would have been doomed to suffer from acute neurodegeneration caused by Spinal Muscular Atrophy (SMA).
“Let me tell you. We treated the very first patient with nusinersen in December of 2011,” De Vivo explains over the phone from his office in upper Manhattan. “It was a pretty exciting moment, when you think about it. We didn’t know for certain whether it was going to be as good as we know it is. It could have been a disaster. That’s just the nature of clinical trials. But it worked out fantastically!”
And why was that? According to De Vivo, beautiful results are the fruit of beautiful science.
“In 1995, we, the medical community, were introduced to the mutation that causes SMA for the first time. Only after this occurred did people increasingly start to say, ‘If we can do something about that mutation, then maybe we can find a perfect treatment for this disease.’ ”
“Enter people like Adrian Krainer who are masters of RNA splicing...”
“Lo and behold,” said De Vivo, a path to a drug for SMA patients could suddenly be seen.
A young scientist's unforeseen path
“In 1999, it—Spinal Muscular Atrophy—was a disease I knew almost nothing about,” Professor Krainer admits with a bashful shake of his head.
He’s sitting in an armchair at Cold Spring Harbor Laboratory’s historic library. A bust of Francis Crick, co-discoverer of the structure of DNA, peers over his left shoulder with stoic indifference. It’s easy to imagine the Nobel laureate’s likeness is actually listening in, subtly turning an ear toward our conversation even as his polished granite eyes pretend to browse titles on a nearby bookshelf.
“It was 1999, and I was invited to attend a workshop at the NIH's National Institute of Neurological Disease and Stroke.”
Krainer was asked to attend the workshop, despite his lack of knowledge on SMA, because he was internationally recognized as one of the world’s leading experts on the molecular process known as “splicing”—a process, his peers had just determined, that plays a critical role in causing SMA.
“The NIH workshop was a watershed moment for me,” Krainer explains. “The splicing errors these people were observing were so obviously similar to what I had been studying.”
So at that meeting in Washington DC, surrounded by experts of a disease he barely understood, Krainer decided to start blazing a path that eventually would lead to a new drug and many lives saved.
How did Krainer reach that point? How does one become an expert on something other scientists were just figuring out?
It was pure serendipity
That’s according to Nobel laureate Richard Roberts—a man whose decades of work in the lab have certainly taught him a thing or two about serendipity...and Adrian Krainer for that matter. In 1977, 20 years before Krainer had his epiphany, Roberts and Phillip Sharp had one of their own that changed the history of the life sciences. In different labs at different institutions, they co-discovered the process of RNA splicing.
According to Roberts, he never intentionally set out to discover RNA splicing. Instead, that big discovery was the accidental fruit of a search for other things—research that required two attributes above all: curiosity and an open mind.
“Why should we celebrate this aspect of scientific discovery?” science writer Peter Tarr recently asked Roberts. They were sitting in Roberts’ office at New England Bio Labs, where he has served as Chief Science Officer (CSO) for the past 25 years.
“Why should we celebrate...”
“Serendipity?” Roberts provided.
“Well, yes, but also the patience that is so characteristic of scientists. It takes time. It’s not a straight line.”
“No, never. Never. And if it was, it would be rather boring,” Roberts cut in. He was grinning at this point. “And I don’t think you’d get particularly talented people to come along and do it.”
— James Watson, Nobel laureate and co-discoverer of the double-helix structure of DNA
Dr. Richard Roberts: why curiosity-driven research is so important.
War. What it's good for
To really press his point home, Roberts provided an example from his own mark on history.
“I mean, think of Nixon’s War on Cancer! If it wasn’t for the War on Cancer there never would have been a biotech industry. Really! And we would have never discovered splicing.”
“But did we get an answer to cancer?” he asked. “No...not at all.”
What the war on cancer did do was provide an inspiring puzzle for some of 1970s' brightest and most ambitious scientists. Among them were Roberts and Sharp.

Richard Roberts (l) and Phillip Sharp (r)
CSHL’s Jim Watson was among the scientific sages who realized cancer was a genetic illness, at its roots. Early in the cancer war, though, there was no way to easily study genes, or even know where specific genes were located on chromosomes. Roberts proposed to use a newly discovered class of proteins called restriction enzymes to cut the enormously long DNA molecule into manageable bits that could then be mapped, if slowly, using a manual method. Similarly, Sharp had used the first of such enzymes to sequence parts of the genome of a cancer-causing virus.
One of nature's greatest inventions
Both scientists applied their skills to a basic mystery that molecular biology was then tackling: When an activated gene’s “message” is copied from the original DNA into RNA—the first step in making a protein—how does the RNA message actually form?
Using powerful electron microscopes, Roberts and Sharp both noticed that RNA messages were not precise copies of genes. As they were processed in the cell nucleus, they formed odd shapes and eventually became looped and then broke into smaller pieces, as seen in the background image on this page.
Independently, using some of the same know-how and tools, Sharp's lab (at MIT) and Roberts with his team (at CSHL) soon concluded that a preliminary, raw copy of a gene first had to be edited, or “spliced,” before it could be used to direct protein production.
The research baton is passed
Roberts and Sharp were acknowledged for the discovery of RNA splicing with a Nobel Prize in Physiology or Medicine in 1993. Just before then, Roberts found himself in a position at CSHL to promote a new generation of young scientists to the forefront of efforts to more completely understand splicing.
“For me, I look for enthusiasm,” Robert’s explained.
“If people are enthusiastic...”
Attending a large meeting on RNA splicing in the 1980s, Roberts had been impressed with a presentation made by a grad student from Harvard named Adrian Krainer with a clear passion for splicing research.
Fortunately, Cold Spring Harbor Laboratory was then in the midst of designing a program that would help spur promising postdoctoral researchers. It came to be called the Fellows program, and, on Roberts’ advice, Adrian Krainer became the very first in a distinguished line of CSHL Fellows that also includes Nobel laureate Carol Greider.
A podcast about Adrian Krainer. See all Base Pairs podcasts.
CSHL's president on investing in young scientists.
"Relentless pursuit"
Under Roberts’ tutelage, Krainer was able to flourish, beginning what he describes as a “relentless pursuit of RNA splicing” that continues to this day.
In the early 1990s he made his first breakthroughs, identifying two proteins that would prove important in regulating the splicing process. Over time, more than 200 factors would be identified by labs around the globe.
And when that 1999 NIH workshop rolled around—with experts on Spinal Muscular Atrophy vaguely gesturing at examples of splicing in action—Adrian Krainer suddenly realized how all of his hard work might apply.
Serendipity had turned his scientific journey into the perfect key for a very specific lock. It would take more than a decade still, but he’d beat that disease.
Rich Roberts describes his first impressions of protégé Adrian Krainer.
Chapter 3
A disease of helplessness
Imagine that when you’re born, there is a hand lightly pressing on your chest. At first, it’s difficult to notice; perhaps it only stops you from looking around a room and maybe you have a little less interest in reaching for things. But as time goes on, that hand grows larger and presses down harder. Normally exploring limbs are leaden and breath is pushed from seemingly tiny lungs. You may never learn to sit up. Life with Spinal Muscular Atrophy is not a comfortable one, and if a child is born with a particularly severe version of the genetic disease, it’s a life that won’t last long.
A decade ago, Type 1 SMA would claim a staggering 90 percent of the children born with the affliction each year. Few would make it to their second birthday. Others, somewhat more fortunate, are born with less severe versions. Children with Type 2 SMA usually survive, but only to live with the weight of their own bodies proving too much for their failing muscles. Many lead a breathless, exhausting existence from the confines of a wheelchair.
That is, at least, how it used to be...
“Take that, SMA!”
Today, you might say that SMA has been “beat.”
Approved in the United States and European Union (Japan, Canada, Australia, Switzerland, and Brazil pending), the drug known as nusinersen compensates for the insidious problems within an SMA patient’s genome.
Sold under the brand name SPINRAZA™, the drug has been hailed as the “first-ever treatment for SMA,” and a “huge win” for molecular biology. In fact, SPINRAZA™ was named the "best biotechnology product of 2017" by the prestigious Galien Foundation.
“There has never been a disease-altering therapy for a neurodegenerative disease,” molecular neuroscientist J. Paul Taylor of St. Jude Children's Research Hospital excitedly told Science a few days before the drug became available. (Taylor is not involved with the drug or with the companies behind it.)
But just as the drug’s inventors did, before you can understand how this miracle drug works, you first must understand SMA.
A protein we can’t live without
Working with our friends at Youreka Science, CSHL has created a brief explainer video to help you understand SMA and how nusinersen treats it. We begin with what causes SMA.
SMA is caused by a lack of the aptly named “Survival of Motor Neuron” (SMN) protein. This protein is so important that our muscles cannot develop normally without it.
When a person is born without a properly working SMN1 gene, there’s a serious problem, because it carries instructions for muscle cells to manufacture SMN protein. Deprived of the normal source of vital SMN protein, such a person falls back on a second, nearly identical gene called SMN2. But this gene is flawed. It only makes about 10% of the SMN protein needed by our muscles. These people have Spinal Muscular Atrophy. They usually begin to weaken at the very beginning of life, just as their muscles are developing.
Turning to the backup gene
Once the biological cause of SMA was discovered—a catastrophic flaw in the SMN1 gene—researchers quickly realized that a solution was not going to be easy. Even to this day, there are no approved drugs that repair broken genes. But what about that backup gene we just mentioned? If SMN2 were somehow to become an adequate producer of the vital SMN protein, wouldn’t that halt SMA?
Enter Dr. Adrian Krainer. A curiosity-driven researcher, Krainer’s career has been motivated by his interest in the mechanism of a molecular editing process called RNA splicing that is central in the production of proteins. By the late 1990s—as we describe in Chapter 2—Krainer was known as a master of splicing.
Having worked directly under Nobel laureate Richard Roberts—who co-discovered splicing—Krainer was recognized by the research community as a promising candidate to tackle the RNA problem behind SMA.
“Now suddenly here’s a common disease which I’m learning about in which all the patients have the same defect [in SMN2],” says Krainer. “It was really apparent that if we could find a solution, it would apply to all SMA patients.”
Much of the work Krainer had done as a CSHL Fellow and young lab leader would play a major role in the development of nusinersen. But it would take time to secure funding and introduce others in his lab to the idea of fixing the backup gene, SMN2, as a way of helping SMA patients.
A magical discovery
“It was almost magic—the way things ended up working,” says Dr. Frank Bennett, a leader of drug development at Ionis Pharmaceuticals.
However, Bennett, who was instrumental in nusinersen’s development, knows that it was hard work, not magic, that got the drug to the patients who need it.
“Working with Adrian [Krainer] has been one of my most enjoyable collaborations,” he explains. “It was like two streams of basic research coming together.”
Like most basic research, Krainer’s own stream started as a trickle, but grew into a torrent of insights into the molecular drivers of SMN.
In the late 1980s Krainer had sought to isolate the individual components that made splicing happen. “My first real breakthrough,” he recalls, “was to purify a single protein, which is now called SRSF1.”
An excerpt from Base Pairs episode 5: Dr. Krainer explains how an exhausting method to isolate specific proteins helped him make discoveries that later led to a drug for SMA. See all Base Pairs podcasts.
Later, work in his lab with postdoc Akiya Mayeda determined that SRSF1 is a splicing activator.
As if by fate, it was this same essential activator that Krainer and postdoc Luca Cartegni determined is missing when SMN2’s RNA message is spliced, leading to the problematic skipping of one chunk of its message, called exon 7. Soon after, the pair developed a method called ESSENCE to correct this skipping problem.
And that’s what got Bennett’s attention. There were plenty of kinks to be worked out of ESSENCE, and in 2004, Krainer and the Ionis drug developer worked to optimize the system.
Dr. Adrian Krainer in his lab
Pleasant surprises
There were more surprises still in store. After Krainer’s identification of SRSF1 as the missing splicing activator that caused exon 7 to be skipped—impairing the SMN2 “backup” gene—he realized that the corrective molecule used in ESSENSE was also able to correct exon skipping when stripped down to a short, “naked” sequence of RNA. Scientists call these tiny sequences antisense oligonucleotides, or ASOs. This was key in the design of the drug called nusinersen. Efforts began to synthesize an ASO with the greatest ability to promote the inclusion of exon 7, that missing part of the SMN2 gene’s message that prevented it from making normal amounts of SMN protein.
From there, things moved quickly. In April 2008, results from mouse trials proved the treatment could correct for SMN2’s major flaw in living cells. Subsequent research even showed that the drug could reverse symptoms of Type 1 SMA, the often fatal form of the disease that we mentioned at the start of this chapter. Soon, Ionis received permission to start testing the drug in people.
By 2015, several pivotal Phase 3 trials were in progress, and one—a trial for infants suffering from SMA—came to a sudden halt. However, it wasn’t disaster that struck. Instead, it was success.
Dr. Krainer describes a key moment during clinical trials that exemplifies nusinersen's success in treating SMA.
Those results were indeed “good enough to convince the FDA,” and in December 2016, the once-experimental treatment became commercially available to SMA patients for the first time.
Meet a superhero
Of course, while wonderful results and FDA approval are successes of their own, Krainer would argue that his biggest success is all the young lives that get to continue thanks to basic research driven by curiosity about how cells work. This was a big dividend.
“When you do the research, you always hope that it's going to have a beneficial impact, but it always seems like that is a distant possibility,” Krainer explains. “It's something you strive for, and now it seems to actually be happening and it's like a dream come true, very rewarding."
Emma Larson was treated with nusinersen during clinical trials. Today, as she and her parents visit Dr. Krainer at Cold Spring Harbor Laboratory, she's stronger than ever!
Credits
Product of CSHL Communications Department
Written by
Brian Stallard
Edited by
Peter Tarr, Sue Runkowski, and Philip Renna
Produced by
Sue Runkowski and Brian Stallard
VP Communications
Dagnia Zeidlickis
"Sink or Swim" Base Pairs podcast episode 5
Written by Andrea Alfano and produced by Brian Stallard
Photo and video material (CC BY):
Biogen, Inc.
Ionis Pharmaceuticals
New England Biolabs, Inc.
National Cancer Institute
Photo and video material (CC BY-NC):
The Larson family
Nikita No Komment
Robert Harding, Hope4Cameron, & The Fast Movement
Stephen Clark & WXYZ-TV Detroit
Additional video & photography ©:
CSHL Communications Department
CSHL Archives
Youreka Science
Kathy Kmonicek, 2016/CSHL
T5G Productions & Christopher Gazzo
Special thanks to:
Emma, Dianne, and Matt Larson
Sir Richard Roberts, Ph.D.
Darryl C. De Vivo, M.D.
C. Frank Bennett, Ph.D.
Professor Adrian Krainer, Ph.D.